Volume 13 - Year 2025 - Pages 47-57
DOI: 10.11159/ijepr.2025.006
Inhalation Risk Assessment of Formaldehyde and PM2.5 from Fragrance Product Emissions in Indoor Environments
Srideep Dasari1, Meenakshi Kakara2, Sneha Maloth2, Shivram Karthikeyan2, and Keerthi Katam2
1Mahindra University, Department of Chemistry,
École Centrale School of Engineering, Hyderabad, Telangana, India, 500043
srideep.dasari@mahindrauniversity.edu.in
2Mahindra University, Department of Civil Engineering,
École Centrale School of Engineering, Hyderabad, Telangana, India, 500043
meenakshi20ucie024@mahindrauniversity.edu.in;
sneha20ucie036@mahindrauniversity.edu.in; se22ucie007@mahindrauniversity.edu.in;
keerthi.katam@mahindrauniversity.edu.in
Abstract -
The objective of this study is to assess the indoor air quality and inhalation exposure risk during the use of perfumes. The air quality parameters, such as Formaldehyde (HCHO), particulate matter (PM10, PM2.5, PM1.0), carbon monoxide (CO), carbon dioxide (CO2), total volatile organic compounds (TVOC), ozone (O3), and air quality index (AQI), are measured indoors after spraying perfumes. The average values of thirteen perfumes after spraying three times are as follows: PM2.5 concentration is 97.2 µg/m³, HCHO level is 3.7 ppm, and CO2 concentration is 526.6 ppm. The TVOC stands at 5.18 ppm, while the AQI is 210. Additionally, the PM10 level is 68.8 µg/m³, CO (carbon monoxide) is at 11.15 ppm, O3 (ozone) is measured at 0.69 ppm, and PM1.0 is 30.96 µg/m³. These readings signify high concentrations of different pollutants emitted into the air, which may contribute to indoor air contamination and health effects upon frequent exposure. The high concentration of PM2.5 and PM10 may lead to respiratory discomfort, such as asthma and impaired lung function. HCHO induces irritation in the eyes, nose, and throat, and long-term exposure can result in aggravated respiratory conditions. The presence of high TVOC levels can cause headaches, dizziness, etc. High levels of CO can hinder the process of oxygen supply in the body, which can result in cardiovascular problems. The research identifies a high possible carcinogenic hazard related to the use of the perfumes tested, as indicated by HQ values greater than 1 for formaldehyde. These results emphasize the need for regulation and surveillance of fragrance ingredients in order to maintain consumer safety.
Keywords: particle count, perfumes, personal cloud, TVOC
© Copyright 2025 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2025-04-10
Date Revised: 2025-09-29
Date Accepted: 2025-10-12
Date Published: 2025-10-15
1. Introduction
Indoor air quality (IAQ) is a very simple measure of human health and well-being, given that human beings spend approximately 90% of their time indoors [1]. Indoor air pollution has been linked with an extremely wide range of adverse health outcomes, from respiratory illness to cardiovascular, allergic, and even cancer [2]. The items, such as deodorants, lotions, hair dyes, cosmetics, shampoo, and makeup products, fall under the category of personal care products (PCPs). There is a growing concern regarding PCPs due to their adverse impact on individuals, public health, and the environment in general, and they are employed in everyday life for beautification and maintenance. PCPs contain a large number of chemicals and compounds that can be harmful. For example, PCPs generally have phthalates, parabens, triclosan, and artificial scents [3]. Such compounds can be absorbed into the human body through skin, breathing, or hand-mouth actions. Inside the body, certain compounds can become endocrine disruptors, which can lead to hormonal changes, an imbalance in reproduction, higher chances of specific types of cancers, and multiple health issues [4]–[6]. Among the various indoor air pollution causes, consumer care products, including perfumes and deodorants, have gained significant interest due to their widespread usage and release of volatile organic compounds (VOCs), which are easily able to pollute [7].
Consumer care products release complex mixtures of chemicals, such as terpenes, alcohols, esters, and aldehydes, and have direct effects on indoor air composition and can cause chemical interactions leading to secondary pollutants [8]–[12]. In addition to the indoor environment, every individual is surrounded by a personal cloud created by personal emissions like breath, skin secretions, and applied chemicals like perfumes. Research has indicated that people release VOCs continuously into indoor environments, and personal hygiene practices like showering frequency, activity, and product application manage these emissions. For example, people who skip showers or sweat more will have higher emissions of carboxylic acids. This is because ozone (O3) in the air reacts with oils and grease on skin, hair, and clothing. As a result, O3 is removed from the air but produces by-products such as acetone and aldehydes [13], forming a human oxidation shield. This is beneficial as it prevents inhalation of O3 and reduces its effect on the lungs. When perfumes and deodorants are applied, this human antioxidant shield is disrupted, as the application of perfume produces emissions of a mixture of VOCs such as formaldehyde, benzene, toluene, limonene, linalool, and various esters, which react with O3, altering interactions with the skin and the chemistry of indoor air [14]–[16]. The ozonolysis chemical reaction between VOCs and indoor O3 leads to the formation of secondary organic aerosols (SOAs), formaldehyde, and fine particulate matter (PM1.0). The resultant SOAs or their precursors can penetrate deeper levels of the upper respiratory pathway and are potentially harmful due to their fine size [8], [9], [17]–[19]. The objective of this study is to assess the indoor air quality after spraying the perfume and assess the inhalation exposure risk of formaldehyde and PM2.5 during the use of perfumes.
2. Methodology
2. 1. Measurement of Indoor Air Pollutants
Thirteen different perfumes from the market were chosen for analysis to assess their effect on indoor air quality, representing both men's and women's fragrances from various brands. The perfumes were anonymized and labelled as P1 to P13. Tests were conducted under a closed indoor condition with poor external ventilation in order to replicate typical usage conditions. The ambient air quality inside the indoor room was measured as a baseline. Five sprays of each perfume were released into the enclosed room, and air quality parameters were measured two minutes after spraying. Measurements were taken using a high-resolution indoor air quality monitor that was equipped with specialized sensors, each selected for resolution, sensitivity, and accuracy appropriate for low-level indoor exposure. Parameters being monitored were Formaldehyde, PM1.0, PM2.5, PM10, Total Volatile Organic Compounds (TVOCs), Carbon Monoxide (CO), Carbon Dioxide (CO₂), Ozone (O₃), particle number, and the Air Quality Index (AQI).
2.2. Exposure risk assessment
The carcinogenic risk assessment was approximated using the United States Environmental Protection Agency (USEPA) method of risk assessment [20]. The carcinogenic risk was approximated by the Lifetime Cancer Risk (LCR), which is a quantification of the chance that an individual will develop cancer in their lifetime as a result of prolonged exposure to a carcinogen such as formaldehyde. The LCR was calculated from equations 1 and 2.
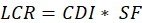
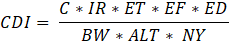
Where CDI is the chronic daily intake (mg/kg/day) and SF is the slope factor for formaldehyde, set at 0.0455 (mg/kg/day)-1 and 1.1 (mg/kg/day)-1 for PM2.5 from the USEPA's Integrated Risk Information System (IRIS). In equ.2, C represents formaldehyde concentration (mg/m³), IR the inhalation rate (16 m³/h), ET the duration of exposure (5 min/day = 0.083 h/day for formaldehyde and 0.5 h/day for PM2.5), EF the frequency of exposure (365 days/year), ED the duration of exposure (50 years), BW the body weight (55 kg), ALT the average lifetime (70 years), and NY the number of days of exposure a year (365). The acceptability of LCR values adheres to WHO standards, in which values <10⁻⁶ are considered negligible risk, 10⁻⁶–10⁻⁵ potential risk, 10⁵–10⁻⁴ probable risk, and >10⁻⁴ certain risk.
Non-carcinogenic risk was estimated through the use of the Hazard Quotient (HQ) given in equation 3, and EC is the exposure concentration computed based on equation 4.
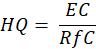
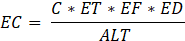
Where RfC is the reference concentration (9.8 × 10⁻3 mg/m³), and EC was calculated from the same parameters. An HQ > 1 indicates potential non-carcinogenic effects on health, whereas an HQ < 1 indicates an insignificantly small risk.
3. Results and discussion
The measurement of air quality parameters after spraying the thirteen various perfumes (designated as P1–P13) in a controlled indoor environment indicates considerable variation in pollutant concentrations from the baseline. Baseline readings before perfume application indicated low pollutant levels: PM2.5 at 28 µg/m³, formaldehyde (HCHO) at 0.02 ppm, CO₂ at 460 ppm, TVOC at 0 ppm, AQI at 40, and low PM10, CO, and ozone levels. Nevertheless, when the perfumes were sprayed, these values exhibited different levels of increase in all samples, which implies a direct contribution of perfume components to indoor air pollution.
3.1 Inorganic gaseous pollutants
The concentrations of the inorganic gaseous pollutants (carbon monoxide, carbon dioxide, and ozone) released via perfumes are given in Figure 1. The CO₂ concentrations for the 13 different perfumes ranged from 441.8 ppm to 697 ppm across the different samples, with an average CO₂ concentration of 526.6 ± 59.1 ppm. Twelve of the perfumes were associated with increases in CO₂ concentrations, averaging +66.6 ppm. One perfume did, however, show a very steep increase up to a maximum of 1051 parts per million for P9, indicating a significant contribution of compressed CO₂ propellant. These results demonstrate that perfumes can be direct sources of CO₂ concentrations indoors, and not just the slower oxidation of VOCs that generates CO₂.
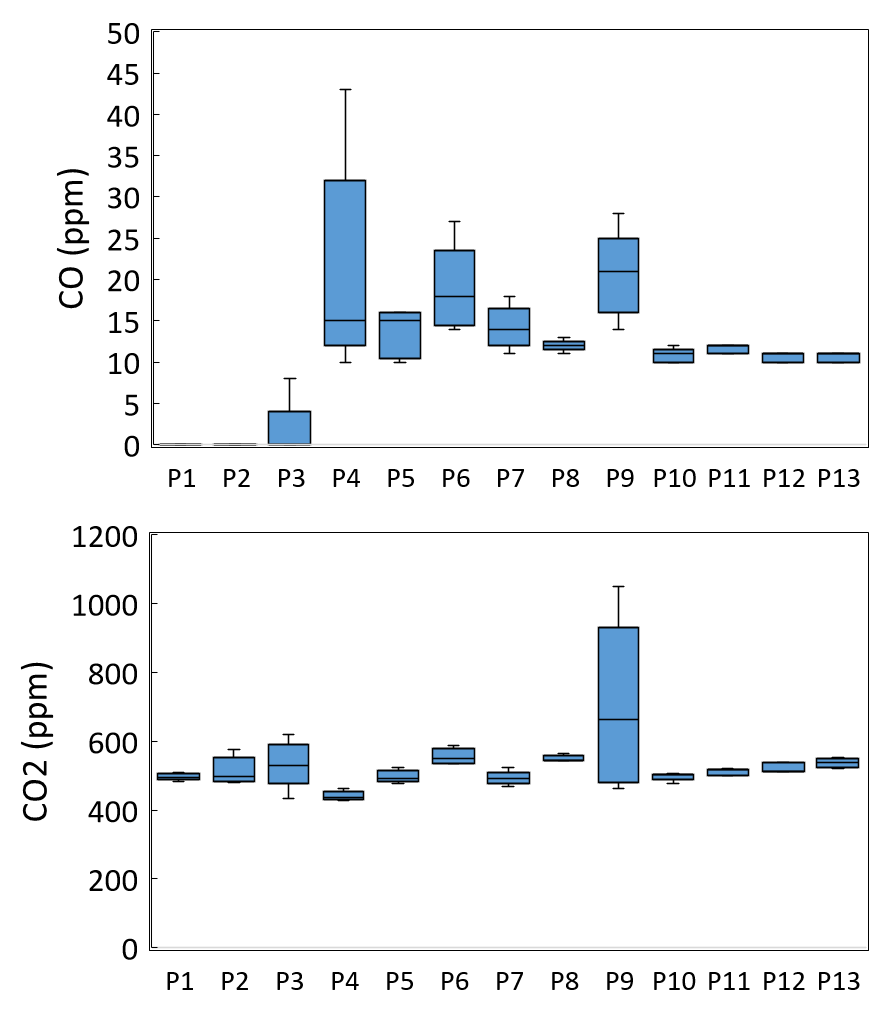
The spraying of perfume resulted in CO concentrations varying between 0 and 20.6 ppm, with a corresponding average level of 11.2 ± 7.0 ppm. Ten out of the thirteen perfumes used demonstrated an observable increase in CO level. The higher levels (~20 ppm) imply that a negligible amount of CO is being produced from VOC oxidation processes that generate incomplete combustion byproducts such as CO, even without any flame present. Variability was also seen, with two perfumes not generating any carbon monoxide (CO), which might be due to the differences in chemical formulation or in the quantity of alcohols and/or terpenes.
Perfume sprays also raised ozone levels above the background concentration to a range of 0.02 to 3.846 ppm and an average of 0.69 ± 1.01 ppm. Eleven of the perfume sprays showed a measurable ozone rise, and there were some above 3 ppm to fall within the abnormally high range of indoor concentrations. Even though ambient ozone levels were low before spraying (0.02 ppm), perfumes such as P4 (9.99 ppm), P11 (1.69 ppm), and others showed sharp O3 spikes. The measured inorganic gases are typically stable indoors, but upon the application of fragrant consumer products add VOCs, including formaldehyde, terpenes, and aromatic hydrocarbons. VOCs themselves exhibit increased chemical reactivity relative to inert gases and may participate in photochemical as well as radical-mediated reactions with ozone, hydroxyl radicals, and nitrogen oxides. Resulting reactions may create secondary pollutants, modify the oxidative capacity of indoor environments, as well as temporarily modify the chemical composition of the air. In general, perfumes do not contain O3; therefore, the increase in the indoor O3 levels might be due to the secondary reactions between VOCs in the perfume, such as terpenes and background O3, generating a series of new pollutants, including the secondary ozone [14].
3.2 Volatile organic compounds
The levels of formaldehyde and TVOCs released after spraying perfumes are given in Table 1. The maximum level of formaldehyde that can be measured by the air quality meter used in this study is up to 5 ppm, and in particular, several perfumes exhibited HCHO levels of 5 ppm (P1, P2, P3, and all applications of P4, P5, P6, and P9) that reached or exceeded common indoor limits. According to WHO guidelines, the indoor exposure limit for formaldehyde is around 0.1 mg/m³ (0.08 ppm) for short-term (30-minute). In indoor settings, formaldehyde is one of the most well-studied VOCs and is commonly associated with perfumes, fragranced products, and secondary reactions with terpenes. Despite its low usage, formaldehyde continues to be a concern among indoor air pollutants as it is still emitted from personal care products, furniture, and building structure materials. The indoor formaldehyde values above 100 μg/m3 were detected in newly renovated residences, schools, and offices [21]. Its health impacts are alarming, being classified as a human carcinogen and showing evidence of an association with nasopharyngeal and leukemic cancers. Some perfumes may contain HCHO in the formulation, which can directly contribute to the indoor levels. Furthermore, even with lower concentrations, formaldehyde acts as a respiratory irritant, contributing to exacerbation of asthma, allergic reactions, and mucosal irritations. In indoor spaces, levels of formaldehyde are also influenced by microclimatic factors, where higher temperatures can increase indoor emissions and exposure risk when ventilation is poor. Due to its toxicological characteristics, persistence in indoor environments, and formation from reactions involving fragrance-related compounds, it continues to be a significant volatile organic compound linked to indoor air quality and public health concerns.
Table 1. Average concentrations of Organic gaseous pollutants (formaldehyde and TVOC) released by perfume samples
|
Perfume |
HCHO (ppm) |
TVOC (ppm) |
|
P1 |
2.39 ± 2.2 |
4.06 ± 1.9 |
|
P2 |
2.40 ± 2.2 |
5.55 ± 0.1 |
|
P3 |
2.34 ± 2.2 |
5.62 |
|
P4 |
5.00 |
5.62 |
|
P5 |
2.04 ± 2.4 |
5.62 |
|
P6 |
5.00 |
5.62 |
|
P7 |
5.00 |
5.62 |
|
P8 |
0.45 ± 0.1 |
5.62 |
|
P9 |
5.00 |
5.62 |
|
P10 |
3.82 ± 1.9 |
3.91 ± 0.9 |
|
P11 |
5.00 |
5.61 |
|
P12 |
5.00 |
3.83 ± 0.8 |
|
P13 |
5.00 |
5.07 ± 0.7 |
TVOC levels for all perfumes increased from 0 to stable levels of approximately 5.62 ppm for the majority of samples, indicating significant VOC loading. Fragrances are among the major indoor volatile organic compound emission sources, emitting compounds such as terpenes (e.g., limonene, linalool, α-pinene), aldehydes (e.g., formaldehyde, benzaldehyde), aromatic hydrocarbons (e.g., benzene, toluene, xylene), and esters. While these compounds contribute to desired scents, they can also build up in indoor air, especially in unventilated spaces, and serve as indoor pollutants. The reported concentrations of some of these species range considerably. A recent study on five fragranced personal care products detected more than 200 different VOCs, whereby the emission factors varied between 2 as well as 964 mg/g [22]. Interestingly, it was also established that when VOCs react in the presence of indoor ozone, they produce oxidized vapors as well as promote ultrafine particle formation (new particle formation, NPF), consequently resulting in ultrafine particle number concentrations ranging about ~34,000 to ~200,000 cm⁻³ [22]. In another study investigating VOC emissions due to indoor use of fragrance diffusers, researchers established that the essential oils and fragrance volatilizing liquids emit compounds as ethanol (19.2 – 40.5 ppb), propylene glycol (273.4 – 527.9 ppb), and substituted benzaldehydes (3.8 – 12.4 ppb); further, emission rates depended significantly on temperature as well as volatilization mode, where VOC by-product emissions kept rising when temperatures or wick heating was increased [23]. In addition, some studies have detected benzene at levels of ~29 μg/m³, toluene at levels around ~87 μg/m³, and formaldehyde at levels ~106 μg/m³ in school environments. Acetone and terpenes may also be emitted in homes, with measurements between a few μg/m³ and several hundred μg/m³ [24], [25]. In addition to direct emissions, fragrance terpenes may react with ozone in indoor settings to generate secondary pollutants such as formaldehyde and ultrafine particles, which may increase exposure risk [26]. Epidemiological studies have identified a range of health consequences to chronic indoor VOC exposures, indicating that benzene is strongly associated with leukemia and low birth weight, toluene & p-dichlorobenzene with asthma, and chlorinated hydrocarbons and esters with neurological and cardiac effects [27]. National surveillance studies also indicate that indoor VOC levels in homes throughout Europe, many deriving from perfumes and personal care products, are equivalent and associated with risk [28]. Overall, perfumes represent an important and potentially underestimated source of indoor air pollution. VOCs emitted from perfumes not only pose direct irritant and potentially toxic effects, but also can be precursors to secondary contaminants that can also be harmful. Continuous monitoring and potential interventions on indoor fragrance exposure should be pursued.
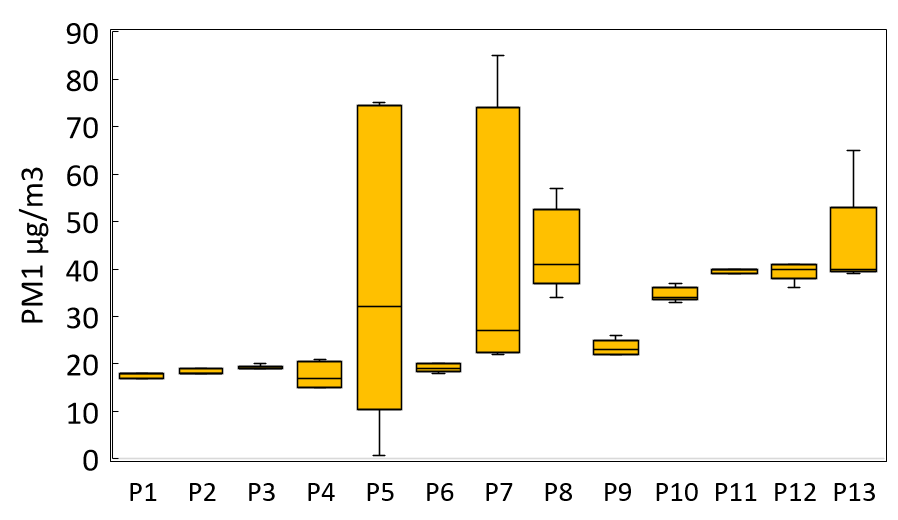
3.3 Particulate Matter (PM) and Particle Count
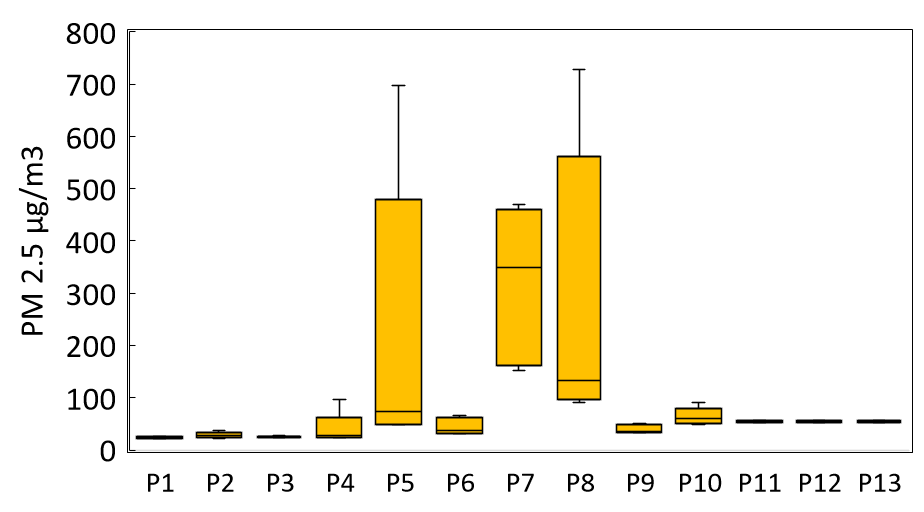
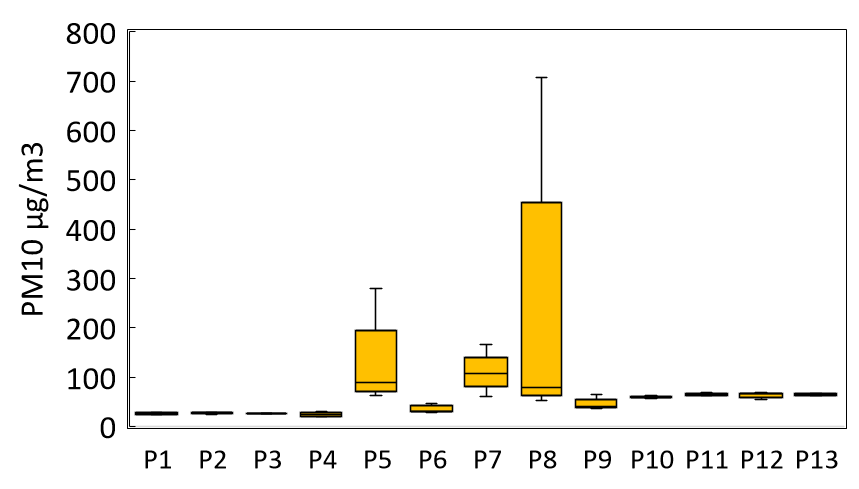
The concentrations of the different PM released via perfumes are given in Figure 2. The application of perfume resulted in substantial increases in concentrations of particulate matter. Perfumes such as P5, P7, and P8 recorded exceptionally high PM2.5 values (698, 470, and 728 µg/m³, respectively), which far exceed the WHO’s indoor air quality guideline of 25 µg/m³ for 24-hour exposure. PM1.0 values ranged from 17.6 to 45.0 µg/m³, with some samples exceeding 40 µg/m³. The concentrations of PM10 showed an even greater range, spanning up to 700 µg/m³, with an average of approximately 69.0 µg/m³. The highest PM10 concentration values indicate a considerable mass of particulate matter is emitted, likely due to both primary aerosol droplets and secondary organic aerosol processed in the surrounding air. The findings indicate a substantially higher amount of particulate matter, both fine and coarse, following the application of perfume sprays in enclosed spaces.
The quantification of particles across all three size ranges of interest, from ultrafine to the coarse forms (>0.3µm to >10µm), recorded a prominent rise in the number of particles (Table 2). Specifically, for the fine fraction of particulate matter of size >0.3µm, maximum values of measurements were within 3517 to and above 15,000 particles/cm³, indicating perfumes to be a prominent source of ultrafine particles. The intermediate size ranges also of >0.5µm, >1.0µm, and >2.5µm showed comparable distribution profiles, wherein a maximum number of particles was above 3825, 902, and 164 particles/cm³, respectively. Similar results were reported where they found that 82-99% of aerosol particles from nine different indoor perfumes and deodorants were less than 0.3 µm in size [29]. For the larger size ranges (>5 µm and >10 µm), despite being found to have a lower frequency of distributions, their presence indicates that perfumes have the potential of producing the entire size distribution spectrum of aerosols from the ultrafine to the respirable coarse particles.
Table 2. Average concentrations of ultrafine particles released by perfume samples
|
>0.3 |
>0.5 |
>1.0 |
>2.5 |
>5 |
>10 |
|
|
P1 |
4091.2 |
437.2 |
96.2 |
7.6 |
1.8 |
0.2 |
|
P2 |
4297.6 |
658.6 |
88.2 |
7.4 |
3 |
0.2 |
|
P3 |
4721.8 |
752.8 |
100.2 |
6.4 |
1 |
0.2 |
|
P4 |
3517.6 |
552 |
79 |
6.8 |
1 |
0 |
|
P5 |
6960.2 |
1130 |
384.6 |
70.4 |
22.6 |
6 |
|
P6 |
4441.8 |
772.6 |
156 |
21 |
5.6 |
1.8 |
|
P7 |
5860.2 |
433.8 |
222.2 |
32.6 |
11.4 |
3.8 |
|
P8 |
15028 |
3825.4 |
902.2 |
164.4 |
60.2 |
17.4 |
|
P9 |
5665.8 |
1056 |
175 |
17.2 |
5.2 |
1.6 |
|
P10 |
10163.8 |
1529.2 |
316.8 |
30.6 |
8.4 |
2.6 |
|
P11 |
11879.6 |
2276.6 |
350.8 |
36.8 |
10 |
3 |
|
P12 |
12081.6 |
1847.4 |
375.2 |
39.2 |
10.6 |
3.2 |
|
P13 |
11810.6 |
2149.2 |
369.2 |
36.8 |
11 |
3.2 |
The measured high levels of particulate matter can be accounted for by two major mechanisms: (i) aerosolization of perfume droplets upon application, yielding immediate mass loadings of particles across a wide size range, and (ii) the creation of secondary particles via atmospheric chemical processes, especially through condensation and the creation of secondary organic aerosols from volatile carbons. The relative dominance of ultra-fine and fine particles (namely those above 0.3 µm and 2.5 µm, respectively) is of special interest, as particles within these diameters are capable of reaching the deepest regions of the respiratory tract and thus inducing oxidative injury and inflammation.
From a public health standpoint, the rise in levels of PM2.5 and PM10 measured here exceeds worldwide air quality limits and indicates a possible resultant effect on the lung from perfume usage within enclosed spaces. Additionally, the high-level concentration of ultra-fine particles detected has important systemic ramifications, as clinically relevant ultra-fine particles from the pulmonary compartment can migrate to the circulatory system. In addition, high emissions of particulates are responsible for the creation of haze within enclosed spaces and have atmospheric chemical consequences, since aerosolized sprays ultimately diffuse to the outdoor environment, contributing to long-term particulate air pollution in the urban setting. The results from this study highlight the fact that spray aerosols are an important source of gaseous air pollution agents, including a list of substances, including CO₂, CO, O₃, volatile organics, and particulate matter.
3.4 Air Quality Index (AQI)
The AQI concentrations, calculated from an array of pollutants, exceeded the maximum limit (999) in samples like P5, P7, and P8, indicating harmful indoor air quality (Figure 3).
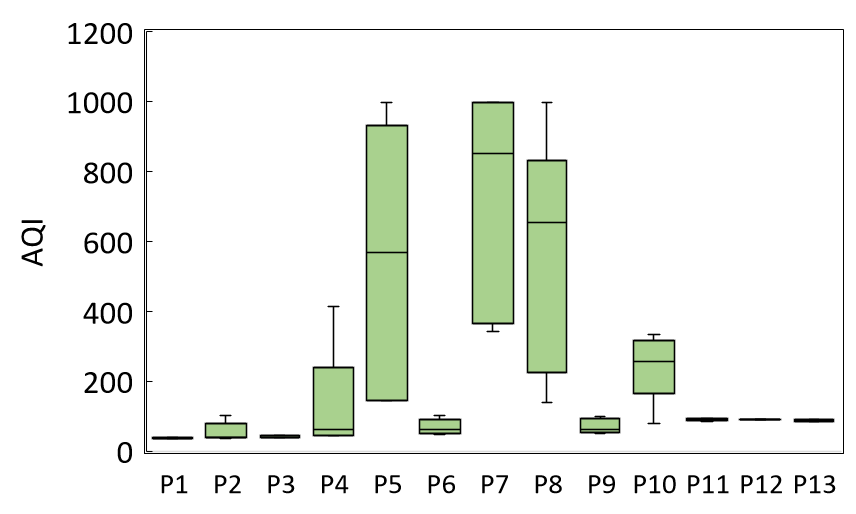
Owing to the cumulative release of VOCs, particles, and ozone from perfumes, there is solid proof that frequent usage in closed places might raise short-term concentrations of harmful compounds and long-term threats to respiratory and systemic effects. The AQI spikes correspond with high input from gaseous emissions (CO₂, CO, O₃, VOCs, formaldehyde) and particulates (PM1.0, PM10, ultrafine particles). Perfumes emit spray airborne VOCs and droplets in immense amounts, raising the level of principal pollution concentrations and secondary pollution from oxidation reactions in the atmosphere. The intensity level of AQI spikes proves perfumes capable of bringing an indoor setting from good to poor air quality in a matter of seconds.
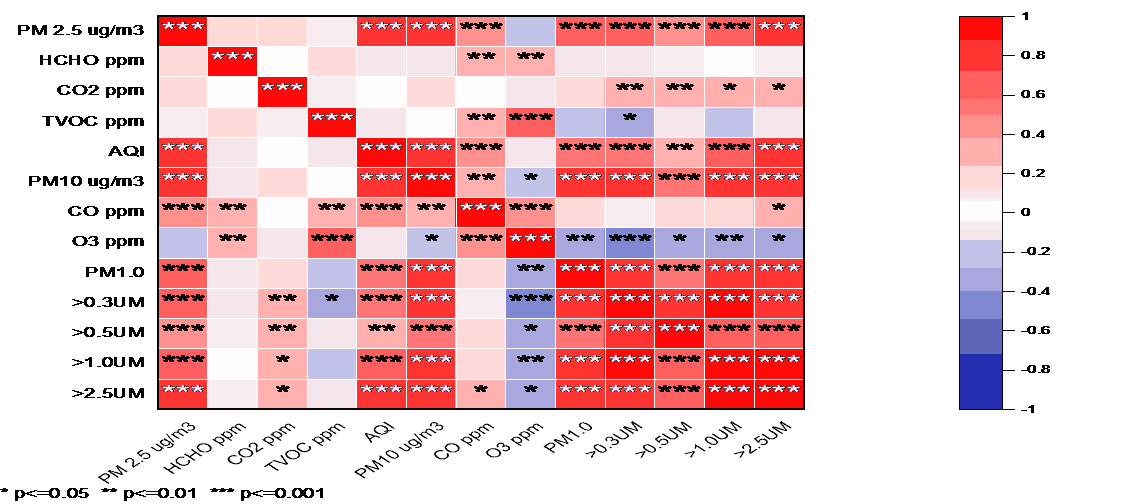
The correlations among the pollutants were investigated via a Spearman correlation matrix (Figure 4). The results showed that there were strong correlations of PM2.5 and PM10 with almost all the other measured variables, such as CO, CO2, TVOCs, AQI, and ultrafine particles. This finding is consistent with the hypothesis that perfumes that emit particulate matter also emit significant amounts of gaseous pollutants. There were statistically significant correlations of PM2.5 (**p < 0.001) with TVOCs, AQI, and PM10, implying that perfumes that emit high levels of PM2.5 are likely to emit high levels of overall air toxicity and perceivable air quality indices. Likewise, TVOCs showed high correlations with AQI and CO, which emphasizes the importance of volatile organic compounds in indoor air quality maintenance. Notably, ultrafine particles showed positive correlations with TVOCs and PM with all size bins (<30 nm, <50 nm, <100 nm, <200 nm), consequently corroborating the hypothesis that perfumes which emit higher overall VOC content tend also to act as a source for airborne nanoparticle nucleation and growth.
Ozone (O₃) is a secondary pollutant that shows moderate correlations with other parameters, and especially with TVOCs and PM10, which could indicate a relationship through photochemical reactions. Negative correlations observed are also of interest; particular TVOC fractions, when correlated with the lower fractions of ultrafine particles, can reflect more complex chemical interactions or dilution interaction effects. Based on the average, the matrix shows strong clustering of pollutants into groups, particularly those related to emissions relevant to combustion sources and VOCs, which supports the plausibility of creating clusters of perfumes based on similar behavioral groups in the context of emissions.
3.5 Formaldehyde inhalation risk
The exposure risk of formaldehyde of thirteen perfume samples shows different levels of health risk, with special emphasis on high carcinogenic and HQ from perfumes shown in Table 3 and Figure 5. Of particular interest, perfumes P4, P6, P7, P9, P11, P12, and P13 all had a formaldehyde concentration of 5 ppm, which corresponds to an ambient air concentration of 6.14 µg/m³.
Table 3. Average values of CDI, LCR, EC, and HQ of formaldehyde
|
Perfumes |
CDI |
LCR |
EC |
HQ |
|
P1 |
0.05 |
0.0023 |
0.007 |
0.7 |
|
P2 |
0.05 |
0.0023 |
0.007 |
0.7 |
|
P3 |
0.05 |
0.0023 |
0.007 |
0.7 |
|
P4 |
0.11 |
0.0048 |
0.015 |
1.5 |
|
P5 |
0.04 |
0.0020 |
0.006 |
0.6 |
|
P6 |
0.11 |
0.0048 |
0.015 |
1.5 |
|
P7 |
0.11 |
0.0048 |
0.015 |
1.5 |
|
P8 |
0.01 |
0.0004 |
0.001 |
0.1 |
|
P9 |
0.11 |
0.0048 |
0.015 |
1.5 |
|
P10 |
0.08 |
0.0037 |
0.012 |
1.2 |
|
P11 |
0.11 |
0.0048 |
0.015 |
1.5 |
|
P12 |
0.11 |
0.0048 |
0.015 |
1.5 |
|
P13 |
0.11 |
0.0048 |
0.015 |
1.5 |
*Units of CDI - (mg/kg/day); EC- (mg/m3)
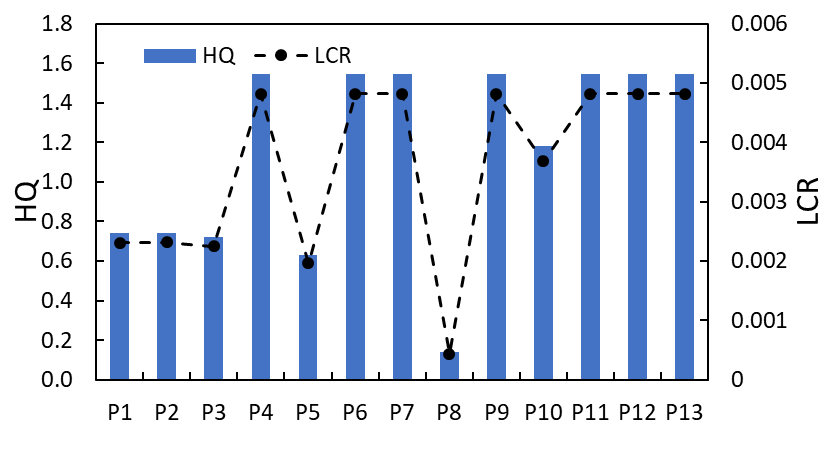
These samples possessed the highest chronic daily intake of 0.11 mg/kg/day and lifetime cancer risk of 0.005, considerably higher than USEPA's limit of 1.0 × 10⁻⁶, signifying an odds of a "definite risk" of developing cancer from long-term exposures. Further, the HQ of these perfumes was well in excess of the safety threshold of 1, presenting high odds of non-carcinogenic effects such as breathing irritation, particularly in sensitive individuals. Even perfumes with a moderate formaldehyde emission (e.g., P10 at 3.8 ppm, HQ=1.2) exhibited a risk above an accepted challenge, while only P8 had both a very low concentration and low HQ (0.1) was well below safety thresholds established for consumers. The estimated Lifetime Cancer Risk (LCR) associated with these exposures registered a range of 0.0020-0.0048 (2-5 ppm HCHO), which was substantially higher than the USEPA standard that indicated a "definite risk" of cancer due to chronic inhalation. The exposure scenario applied here assumed an average daily exposure time of 5 minutes, which is equivalent to the normal time for application of personal care items, like perfumes. Formaldehyde, once released into indoor environments, gets dissipated faster in ventilated rooms. Nevertheless, increasing the number of times the product is utilized might lead to higher cumulative exposures. For instance, the product's repeated use multiple times in one day might bring about increased indoor formaldehyde levels higher than health-related thresholds of exposure. In effect, the risk assessment indicates higher inhalation threats for increased utilization of personal care products that release formaldehyde. In conclusion, frequent use of perfumes that have a high formaldehyde concentration in indoor environments with poorer ventilation would indicate a significantly increased risk for non-carcinogenic effects such as respiratory irritation and lifetime cancer risk. These results indicate the necessity of a stronger regulatory standard for ingredients in perfumes with higher fragrance levels, and a reduction in their use where possible, and for consumers to be educated to limit their inhalation exposure when applying these products.
3.6 PM2.5 inhalation risk
The risk of inhalation exposure to PM2.5 released from consumer perfumes was characterized using measured particulate matter concentrations (µg/m³ and mg/m³) and assessed against associated risk indicators, including Chronic Daily Intake (CDI), Lifetime Cancer Risk (LCR), and Hazard Quotient (HQ) for thirteen different perfumes (P1–P13) shown in Figure 6 and Table 4. PM2.5 levels varied significantly across perfumes, ranging from 23.8 µg/m³ in P1 to a maximum of 318.2 µg/m³ in P7, which showed considerable variability in total particulate emissions in association with different perfumes. Correspondingly, the CDI values ranged from 0.0025 to 0.033 mg/kg-day, suggesting differences in the quantity of particulate mass inhaled daily under assumed exposure conditions. Notably, HQ values demonstrated a metric of non-carcinogenic effect risk with values below 1 indicating an acceptable risk; values ranged from lower levels of 0.07 (P1) to levels maximizing 0.95 (P7). Particularly high-risk perfumes were P5, P7, and P8 with HQ values of 0.67, 0.95, and 0.86, respectively, and these perfume samples present potential for effects on respiratory health upon frequent use or prolonged exposure.
Cancer risk parameters (LCR and EC) were also elevated within the same high PM2.5 emissions, signifying a notably increased and unquantified lifetime cancer risk based on chronic inhalation of PM2.5 exposure related to a consumer product in limited consumer exposure scenarios. These risk indicators further emphasize the risks posed by fine particulate matter (PM2.5) that can be inhaled acutely and chronically, and the contribution of VOCs and secondary organic aerosols produced from fragrances. In conclusion, the findings indicate that indoor air quality is likely to be severely compromised by certain fragrances that release elevated concentrations of particulate matter (PM2.5), and caution is warranted, especially in environments with inadequate ventilation. Consumers and stakeholders should consider the health concerns, chiefly respiratory and carcinogenic risks associated with particulate matter, from these personal care products.
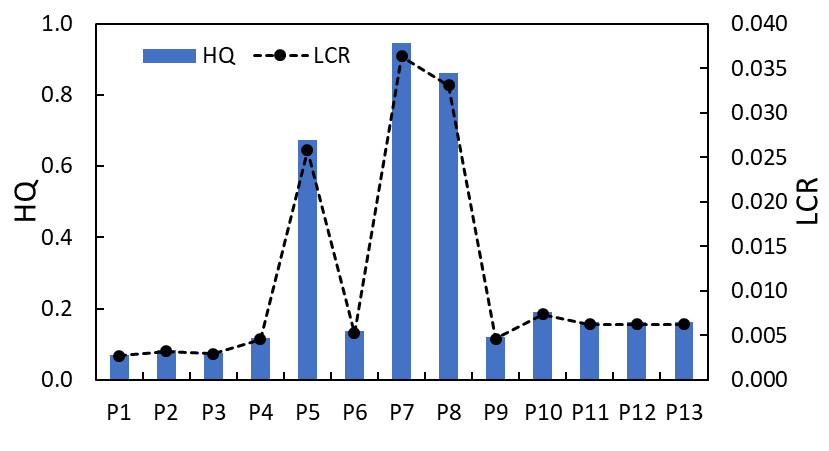
Table 4. Average values of CDI, LCR, EC, and HQ of PM2.5
|
Perfumes |
CDI |
LCR |
EC |
HQ |
|
P1 |
0.002 |
0.003 |
0.0004 |
0.07 |
|
P2 |
0.003 |
0.003 |
0.0004 |
0.08 |
|
P3 |
0.003 |
0.003 |
0.0004 |
0.08 |
|
P4 |
0.004 |
0.005 |
0.0006 |
0.12 |
|
P5 |
0.023 |
0.026 |
0.0034 |
0.67 |
|
P6 |
0.005 |
0.005 |
0.0007 |
0.14 |
|
P7 |
0.033 |
0.036 |
0.0047 |
0.95 |
|
P8 |
0.030 |
0.033 |
0.0043 |
0.86 |
|
P9 |
0.004 |
0.005 |
0.0006 |
0.12 |
|
P10 |
0.007 |
0.007 |
0.0010 |
0.19 |
|
P11 |
0.006 |
0.006 |
0.0008 |
0.16 |
|
P12 |
0.006 |
0.006 |
0.0008 |
0.16 |
|
P13 |
0.006 |
0.006 |
0.0008 |
0.16 |
*Units of CDI - (mg/kg/day); EC- (mg/m3)
The time of PM₂.₅ exposure was set at 30 minutes per day, in between the normal time frame, the finest particulates will likely persist in the airborne phase after the use of personal care products like perfumes. It was proven by numerous studies that the finest particulate matter could remain inside buildings for long periods of time, where the peak concentration happens not long after use, then decreases as time passes. In a non-ventilated room, the reduction in PM2.5 is about 60 % for 10 minutes [30]. Evidence indicates that regular perfume use or use in inadequately ventilated areas can significantly elevate instantaneous inhalation doses, which can enhance acute health effects such as eye, nose, and throat irritation [31]. Alternatively, good ventilation and limited use may reduce total exposure. Nonetheless, even frequent, short-term exposures to high formaldehyde and PM2.5 levels like those in perfumes can contribute to cumulative health risks. The findings emphasize the need for rigorous control and awareness among consumers to avoid inhalation risks involving formaldehyde in cosmetic treatments. Further research is necessary to examine the correlation between emitted pollutants (particulate matter and VOCs) and the associated toxicity mechanisms, considering chemical composition, as well as to investigate strategies for developing safer products.
4. Conclusion
Air quality analysis indicated that usage of perfumes results in direct exposure of the users to a variety of airborne contaminants such as particulate matter (PM), formaldehyde, CO, CO₂, VOCs, and ozone. These contaminants are capable of posing harmful effects to human health in the form of triggering allergies, lung diseases, and even cancer, especially with prolonged use or in poorly ventilated spaces. The Indoor Air Quality index in some of the perfumes exceeded 100, which could mean potentially unsatisfactory air quality contributing to respiratory irritation or other health issues. The research also implies a potential increase in cancer risk associated with all of the assessed fragrances, with a risk quotient greater than 1. However, further research is required to verify the seriousness of actual and possible long-term effects on health. The relevant unknowns would have to revolve around the use of fragrance chemicals, practices of use, and the potential sensitivity of users. It should be remembered that although the existence of pollutants in perfumes and their harmful health impacts have been established, a large percentage of users of such products are not aware of their possible dangers. Perfume users should use them carefully, provide for adequate ventilation, and use products where the composition of the ingredients is indicated. There also have to be stricter regulations about the transparency of the ingredients for public health safety.
References
[1] S. Dimitroulopoulou, M. R. Dudzińska, L. Gunnarsen, L. Hägerhed, H. Maula, R. Singh, O. Toyinbo, and U. Haverinen-Shaughnessy, "Indoor air quality guidelines from across the world: An appraisal considering energy saving, health, productivity, and comfort," Environment International, vol. 178. Pergamon, p. 108127, Aug. 01, 2023. doi: 10.1016/j.envint.2023.108127. View Article
[2] J. L. Domingo and J. Rovira, "Effects of air pollutants on the transmission and severity of respiratory viral infections," Environment International, vol. 187. Academic Press Inc., p. 109650, Aug. 01, 2020. doi: 10.1016/j.envres.2020.109650. View Article
[3] C. J. Gao and K. Kannan, "Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure," Environ. Int., vol. 136, p. 105465, Mar. 2020, doi: 10.1016/j.envint.2020.105465. View Article
[4] S. Chatterjee, S. Adhikary, S. Bhattacharya, A. Chakraborty, S. Dutta, D. Roy, A. Ganguly, S. Nanda, and P. Rajak, "Parabens as the double-edged sword: Understanding the benefits and potential health risks," Science of the Total Environment, vol. 954. Elsevier, p. 176547, Dec. 01, 2024. doi: 10.1016/j.scitotenv.2024.176547. View Article
[5] E. S. Barrett, K. Wadie, K. Getz, P. Greenberg, T. Moore, and A. A. M. Llanos, "Evaluating personal care product use by Environmental Working Group hazard scores in relation to consumers' sociodemographic characteristics, purchasing behaviors, and product safety perceptions," J. Expo. Sci. Environ. Epidemiol., pp. 1-12, Feb. 2025, doi: 10.1038/s41370-025-00751-9. View Article
[6] S. Lim, "The associations between personal care products use and urinary concentrations of phthalates, parabens, and triclosan in various age groups: The Korean National Environmental Health Survey Cycle 3 2015-2017," Sci. Total Environ., vol. 742, p. 140640, Nov. 2020, doi: 10.1016/j.scitotenv.2020.140640. View Article
[7] G. Rádis-Baptista, "Do Synthetic Fragrances in Personal Care and Household Products Impact Indoor Air Quality and Pose Health Risks?," Journal of Xenobiotics, vol. 13, no. 1. J Xenobiot, pp. 121-131, Mar. 01, 2023. doi: 10.3390/jox13010010. View Article
[8] S. Kim, S. H. Hong, C. K. Bong, and M. H. Cho, "Characterization of air freshener emission: The potential health effects," Journal of Toxicological Sciences, vol. 40, no. 5. The Japanese Society of Toxicology, pp. 535-550, Oct. 01, 2015. doi: 10.2131/jts.40.535. View Article
[9] M. Kakara, S. Dasari, M. P. Gundupalli, T. Kangsadan, and K. Katam, "Understanding the Environmental Distribution and Potential Health Risks of Pollutants from Deodorant Products: A Review," in E3S Web of Conferences, EDP Sciences, Sep. 2023, p. 02015. doi: 10.1051/e3sconf/202342802015. View Article
[10] K. Pytel, R. Marcinkowska, and B. Zabiegała, "Investigation on air quality of specific indoor environments-spa salons located in Gdynia, Poland," Environ. Sci. Pollut. Res., vol. 28, no. 42, pp. 59214-59232, Nov. 2021, doi: 10.1007/s11356-020-09860-4. View Article
[11] Z. Kazemi, E. Aboutaleb, A. Shahsavani, M. Kermani, and Z. Kazemi, "Evaluation of pollutants in perfumes, colognes and health effects on the consumer: a systematic review," J. Environ. Heal. Sci. Eng., vol. 20, no. 1, pp. 589-598, Jun. 2022, doi: 10.1007/S40201-021-00783-X/METRICS. View Article
[12] B. You, W. Zhou, J. Li, Z. Li, and Y. Sun, "A review of indoor Gaseous organic compounds and human chemical Exposure: Insights from Real-time measurements," Environment International, vol. 170. Pergamon, p. 107611, Dec. 01, 2022. doi: 10.1016/j.envint.2022.107611. View Article
[13] A. Wisthaler and C. J. Weschler, "Reactions of ozone with human skin lipids: Sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air," Proc. Natl. Acad. Sci. U. S. A., vol. 107, no. 15, p. 6568, Apr. 2010, doi: 10.1073/PNAS.0904498106. View Article
[14] N. Wang, T. Müller, L. Ernle, G. Bekö, P. Wargocki, and J. Williams, "How Does Personal Hygiene Influence Indoor Air Quality?," Environ. Sci. Technol., vol. 58, no. 22, pp. 9750-9759, Jun. 2024, doi: 10.1021/acs.est.4c01698. View Article
[15] A. C. Steinemann, "Fragranced consumer products and undisclosed ingredients," Environ. Impact Assess. Rev., vol. 29, no. 1, pp. 32-38, Jan. 2009, doi: 10.1016/j.eiar.2008.05.002. View Article
[16] M. C. Martini, "Déodorants et antitranspirants," Ann. Dermatol. Venereol., vol. 147, no. 5, pp. 387-395, May 2020, doi: 10.1016/J.ANNDER.2020.01.003. View Article
[17] A. W. Nørgaard, J. D. Kudal, V. Kofoed-Sørensen, I. K. Koponen, and P. Wolkoff, "Ozone-initiated VOC and particle emissions from a cleaning agent and an air freshener: Risk assessment of acute airway effects," Environ. Int., vol. 68, pp. 209-218, Jul. 2014, doi: 10.1016/j.envint.2014.03.029. View Article
[18] T. J. Carter, D. G. Poppendieck, D. Shaw, and N. Carslaw, "A Modelling Study of Indoor Air Chemistry: The Surface Interactions of Ozone and Hydrogen Peroxide," Atmos. Environ., vol. 297, p. 119598, Mar. 2023, doi: 10.1016/j.atmosenv.2023.119598. View Article
[19] J. R. Wells, C. Schoemaecker, N. Carslaw, M. S. Waring, J. E. Ham, I. Nelissen, and P. Wolkoff, "Reactive indoor air chemistry and health-A workshop summary," in International Journal of Hygiene and Environmental Health, Urban & Fischer, Nov. 2017, pp. 1222-1229. doi: 10.1016/j.ijheh.2017.09.009. View Article
[20] P. Foroughi, F. Golbabaei, M. Sadeghi-Yarandi, M. Yaseri, M. Fooladi, and S. Kalantary, "Occupational exposure, carcinogenic and non-carcinogenic risk assessment of formaldehyde in the pathology labs of hospitals in Iran," Sci. Rep., vol. 14, no. 1, pp. 1-12, May 2024, doi: 10.1038/s41598-024-62133-9. View Article
[21] L. Fang, N. Liu, W. Liu, J. Mo, Z. Zhao, H. Kan, F. Deng, C. Huang, B. Zhao, X. Zeng, Y. Sun, H. Qian, C. Sun, J. Guo, X. Zheng, and Y. Zhang, "Indoor formaldehyde levels in residences, schools, and offices in China in the past 30 years: A systematic review," Indoor Air, vol. 32, no. 10. John Wiley & Sons, Ltd, p. e13141, Oct. 01, 2022. doi: 10.1111/ina.13141. View Article
[22] T. Wu, T. Müller, N. Wang, J. Byron, S. Langer, J. Williams, and D. Licina, "Indoor Emission, Oxidation, and New Particle Formation of Personal Care Product Related Volatile Organic Compounds," Environ. Sci. Technol. Lett., vol. 11, no. 10, pp. 1053-1061, Oct. 2024, doi: 10.1021/acs.estlett.4c00353. View Article
[23] W. H. Cheng, Y. C. Chen, and S. Y. Shih, "Volatile Organic Compound Emissions from Indoor Fragrance Diffusers," Atmosphere (Basel)., vol. 14, no. 6, p. 1012, Jun. 2023, doi: 10.3390/atmos14061012. View Article
[24] S. C. Sofuoglu, G. Aslan, F. Inal, and A. Sofuoglu, "An assessment of indoor air concentrations and health risks of volatile organic compounds in three primary schools," Int. J. Hyg. Environ. Health, vol. 214, no. 1, pp. 36-46, Jan. 2011, doi: 10.1016/J.IJHEH.2010.08.008. View Article
[25] C. H. Halios, C. Landeg-Cox, S. D. Lowther, A. Middleton, T. Marczylo, and S. Dimitroulopoulou, "Chemicals in European residences - Part I: A review of emissions, concentrations and health effects of volatile organic compounds (VOCs)," Sci. Total Environ., vol. 839, p. 156201, Sep. 2022, doi: 10.1016/J.SCITOTENV.2022.156201. View Article
[26] B. C. Singer, B. K. Coleman, H. Destaillats, A. T. Hodgson, M. M. Lunden, C. J. Weschler, and W. W. Nazaroff, "Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone," Atmos. Environ., vol. 40, no. 35, pp. 6696-6710, Nov. 2006, doi: 10.1016/j.atmosenv.2006.06.005. View Article
[27] N. Liu, Z. Bu, W. Liu, H. Kan, Z. Zhao, F. Deng, C. Huang, B. Zhao, et al., "Health effects of exposure to indoor volatile organic compounds from 1980 to 2017: A systematic review and meta-analysis," Indoor Air, vol. 32, no. 5, p. e13038, May 2022, doi: 10.1111/INA.13038. View Article
[28] D. Alvarez-Vaca, R. C. Duca, A. Borras-Santos, E. Hardy, M. Creta, C. Eicher, L. Wurth, A. Vergison, and A. Van Nieuwenhuyse, "Surveillance of Indoor Air Concentration of Volatile Organic Compounds in Luxembourgish Households," Int. J. Environ. Res. Public Health, vol. 19, no. 9, p. 5467, May 2022, doi: 10.3390/IJERPH19095467/S1. View Article
[29] J.-F. Bertholon, M.-H. Becquemin, M. Roy, F. Roy, D. Ledur, I. Annesi-Maesano, and B. Dautzenberg, "Particle Sizes of Aerosols Produced by Nine Indoor Perfumes and Deodorants," Int. J. Environ. Monit. Anal. 2015, Vol. 3, Page 377, vol. 3, no. 6, pp. 377-381, Dec. 2015, doi: 10.11648/J.IJEMA.20150306.11. View Article
[30] V. T. Seller, C. D. Brilliant, C. Morgan, S. P. Lewis, J. Duckers, F. A. Boy, and P. D. Lewis, "Anti-perspirant deodorant particulate matter temporal concentrations during home usage," Build. Environ., vol. 195, p. 107738, May 2021, doi: 10.1016/J.BUILDENV.2021.107738. View Article
[31] P. Wolkoff and G. D. Nielsen, "Effects by inhalation of abundant fragrances in indoor air - An overview," Environment International, vol. 101. Environ Int, pp. 96-107, 2017. doi: 10.1016/j.envint.2017.01.013. View Article

