Volume 1 - Year 2012 - Pages 119-125
DOI: 10.11159/ijepr.2012.017
Microbial Growth Control in Diesel by Optimization of Sulphur
N. K. Srivastava*, Nitin K. Nandan
Department of Chemical Engineering, Dr. B. R. Ambedkar National Institute of Technology Jalandhar
G. T. Road, Bypass, Jalandhar-144011, Punjab, India
srivastavank@gmail.com; nitinknandan@gmail.com
Abstract - Long storage of diesel results in the microbial growth causing sludge formation, fuel loss and corrosion in diesel storage tanks. The use of biocides has proven ineffective due to the resistance developed by the microbes as well as excessive concentration of biocides would prove harmful to humans. The purpose of this paper is to optimize the sulphur content in diesel so that the losses due to microbial growth and corrosion are minimal. The samples of 350, 400, 450 and 500 ppm sulphur content were prepared to analyze the corrosion rating and extent of growth of Hormoconisresinae in each sample by measuring the loss in the diesel and formation of sludge. Graphs are plotted to analyze the growth and corrosion in terms of loss/year against sulphur content and the sulphur content is optimized to minimize the total loss. The optimized sulphur content came to be between 380-400 ppm.
Keywords: EuroIII Diesel, Hormoconisresinae, SulphurOptimization, Corrosion Rating, Growth Rating
© Copyright 2012 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
1. Introduction
The petroleum refining industry is one of the largest manufacturing industries in the world spending huge amounts each year on capital equipment, modernization and maintenance, including prevention and treatment of microbial contamination. The continual growth of diesel demand is driven by a number of factors: the superior economy and efficiency of diesel engines; advances in combustion systems and improvements in emission devices including filters for the removal of micro-particulates and NOx reduction systems (Sharafutdinov et al, 2012). The major microbial problem in the industry is contamination of stored products, which can lead to loss of product quality, formation of sludge and deterioration of pipe work and storage tanks, both in the refinery and at the end-user (Gaylarde, 1998).
Reports of such contamination have increased substantially in recent years, probably due to increasing demand for diesel fuel and high quality gasoline and jet fuel and the decreasing sulphur content in the fuels. Crude oil is a mixture of many different hydrocarbons, straight, branched and cyclic aliphatic, aromatic and heterocyclic compounds. The composition varies with the origin of the oil; heavy crudes generally have high carbon, metal and asphaltene content and are less stable chemically than lighter crudes.
The refining process can be divided into four phases:
- 1) Separation,
- 2) Cracking,
- 3) Chemical reactions such as polymerization or alkylation,
- 4) Blending.
Refinery products are mixtures of compounds. Gasoline, for example, contains straight chain and branched-chain. Three major types of fuels are discussed in this article-gasoline, aviation kerosene and diesel, corresponding to increasing heavy petroleum fraction. The fuel that presents the most serious microbial problems is diesel. The amount of sulphur contained in diesel engine fuel should be as low as possible (Aydin and Ilkilic, 2012). The condition required for microbial growth and the method used to monitor and to control this activity are discussed (Christine, 1998).
Diesel, for example, is a hydrocarbon product boiling between approximately 150°C and 400°C, with carbon chain lengths of C15-C22 containing straight chain and branched-chain hydrocarbons, alkenes, naphthenes, aromatics and other compounds. The low volatility of diesel provides larger time and areas of contact between the microbe and the fuel. Diesel engines have high thermal capacity and high fuel efficiency (McClellan et al, 2012). A variety of additives may be used to improve the stability of the fuel; these include compounds such as aliphatic amines, chelating agents, detergents and corrosion inhibitors, some of which can act as a nutrient source for microorganisms. Diesel is the fuel which suffers from the most varied microbial contamination problems. The enhanced affinity to water also contributes to elevated microbial growth in diesel.Rising concerns of the volatile crude oil price, threats to national security and adverse environmental impacts from using fossil fuels are propelling many political mandates, economic incentives and societal investments in alternative fuels (Lin et al, 2013).
1.1. Contamination Problems in Fuel Storage Tanks
Even in the best-kept tanks, microbial contamination is an occasional problem. Microorganisms are usually present in the fuel, but good housekeeping (removal of water and use of biocides) minimizes their growth (Hill & Hill, 1993). Nevertheless, reports of microbial growth in fuel tanks have increased in the last few years and the holding of strategic reserves for long periods has always been problematic (Hartman, 1991).
The presence of bacteria in diesel fuel is the leading cause of engine breakdown. Bacteria and fungi will form insoluble particulate matter that can clog fuel filters, resulting in fuel starving and engine stoppage. They can also corrode metal surfaces, including storage tanks and pumps, and will form organic acids that contribute to fuel instability. Reducing the amount of sulfur in diesel fuel renders the fuel vulnerable to microbial growth. In addition to removing oxygen and nitrogen, the process for removing sulfur from fuel requires introducing water. When fuel becomes contaminated, the water in diesel fuel becomes a breeding ground for bacteria and fungus.
1.2. Nutrients Required in the Microbial Growth and Sources
The most important requirement for microbial growth in fuels is water. This is almost always present, for the following reasons:
- 1) Water dissolved in the fuel can condense on tank walls
- 2) Moisture in the air can enter through floating tank lids or other vents
- 3) Poorly designed tanks do not drain efficiently
- 4) Water may be added as ballast (on ships) or to purge the delivery system.
Oxygen is normally present in sufficient quantities in distillate fuels, and is continually replenished when tanks are refilled. However, even if the fuel becomes anaerobic, it is not protected from microbial attack, since facultative organisms, such as Hormoconisresinae, and anaerobes continue to thrive. The limiting factor to growth is probably availability of minerals, particularly phosphorus, which is generally present at <1ppm in the fuel. Nitrogen and iron may also be important limiting nutrients.
Many laboratory-based studies have shown that fungi grow much more readily in a fuel system containing mineral salts solution as the aqueous phase than with water, or even tank drainage water. However, apart from minerals entering in water or aerial contaminants, many of the additives now used in the fuel industry contain these vital mineral elements, removing one of the factors that limited growth in earlier times.
1.3. Problems Associated with Microbial growth
In diesel fuel, microbial contamination may contribute to aging instability, but in general the most important consequences are microbial induced corrosion of the storage tanks and pipe work, and formation of microbial mats, with the ability to block filters and pipelines, and to increase wear in pumps. According to Irish and Richardson, as little as 1 mg particulates/100mL fuel can cause filtration problems. In addition to microorganisms, these may include dirt, dust, sand, components of other filters such as paper or cotton, pump wear particles, corrosion debris and material removed from tank or pipe linings, such as fibre glass. Corrosion from within tanks and pipelines can be intense when microbial contamination is present.
Current tests however require a minimum of 24 hours and there are still financial implications involving downtime and clean-up operations when contamination is found. The major microorganisms involved in storage tank corrosion are the anaerobic SRB and Hormoconisresinae. Aerobic bacteria may also participate in the process.Fuel storage tanks in ground are especially prone to contamination problems, because of the difficulties of drainage. Since they are hidden from view, it may not be noticed that they are corroded and leaking and thus important environmental pollution can result.
1.4. How Microbes Enter the Fuel
Microorganisms may enter the fuel from the soil, via the air, from polluted wash water, contaminated pipelines, or from the biofilm present on the tank walls, if the latter have not been sufficiently well cleaned. Not all of the organisms are capable of metabolizing hydrocarbons. Some organisms simply survive by catabolism, apart from the hydrocarbons; organisms may gain nutrients from fuel additives, from dirt entering the system, or from the growth of primary colonizing organisms. The hydrocarbon chains most readily utilized are C10-C18.Shorter chain hydrocarbons may actually inhibit growth of some organisms. Different strains of H. Resinae however have been reported as having different optimal chain lengths for growth.
Creosoted matchstick technique found highest yields on n-alkanes C13-C18. The literature shows two isolates from jet fuel and found these to have optimum growth at chain length C11. This may reflect the alkane chain lengths in the substrate from which the fungi were isolated, creosote containing the longer chains from approximately C12-C24.
1.5. Corrosion Problems
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Formation of an oxide of iron due to oxidation of the iron atoms in solid solution is a well-known example of electrochemical corrosion, commonly known as rusting. This type of damage typically produces oxide(s) and/or salt(s) of the original metal. Corrosion can also refer to other materials than metals, such as ceramics or polymers, although in this context, the term degradation is more common.
In other words, corrosion is the wearing away of metals due to a chemical reaction. Many structural alloys corrode merely from exposure to moisture in the air, but the process can be strongly affected by exposure to certain substances. Corrosion can be concentrated locally to form a pit or crack, or it can extend across a wide area more or less uniformly corroding the surface. Because corrosion is a diffusion controlled process, it occurs on exposed surfaces. As a result, methods to reduce the activity of the exposed surface, such as passivation and chromate-conversion, can increase a material's corrosion resistance. However, some corrosion mechanisms are less visible and less predictable.
Diesel is a hydrocarbon with various specifications, for moisture content, sulphur content etc. The tanks containing diesel are generally corroded and hence their internal stresses are elevated compromising the strength and safety of the personnel. Hence regular ultrasound checkups and welding is done, the values of stress at key points are kept minimum (Rajasekar, 2006).
Microbial corrosion, or bacterial corrosion, is a corrosion that caused or promoted by microorganisms, usually chemoautotrophs. It can apply to both metals and non-metallic materials, in both the presence and absence of oxygen. Sulphate-reducing bacteria are common in lack of oxygen; they produce hydrogen sulphide, causing sulphide stress cracking. In presence of oxygen, some bacteria directly oxidize iron to iron oxides and hydroxides, other bacteria oxidize sulphur and produce sulphuric acid causing biogenic sulphide corrosion. Concentration cells can form in the deposits of corrosion products, causing and enhancing galvanic corrosion.
Accelerated Low Water Corrosion (ALWC) is a particularly aggressive form of MIC that affects steel piles in seawater near the low water tide mark. It is characterised by an orange sludge, which smells of Hydrogen Sulphide when treated with acid. Corrosion rates can be very high and design corrosion allowances can soon be exceeded leading to premature failure of the steel pile. Piles that have been coating and have cathodic protection installed at the time of construction are not susceptible to ALWC. For unprotected piles, sacrificial anodes can be installed local to the affected areas to inhibit the corrosion or a complete retrofitted sacrificial anode system can be installed. Affected areas can also be treated electrochemically by using an electrode to first produce chlorine to kill the bacteria, and then to produced a calcareous deposit, which will help shield the metal from further attack.
1.6. Additives: Advantages and Disadvantages
It has been suggested that microbial growth in gasoline occurs only at the expense of additives such as vegetable oil phosphatides, and certainly large-scale microbial contamination is seen only in the presence of organic additives. H.resinae grew in gasolinecontaining 13% alcohol for only 6 days after inoculation, but that changes in the hydrocarboncomposition were, nevertheless, produced. It is also rare to find SRB as gasoline contaminants, but thismay be due to the toxic effect of gasoline hydrocarbons. The presence of various additivesin diesel oil has been held responsible for increased microbial problems, but recent work at UFRGS (Universidade Federal do RioGrande do Sul) suggests that certain new additives have no effect on fungal growth. It is important to test agents intended to improve chemical and physical fuel properties for their potential as stimulators of microbial growth. Utilization of an additive by microbial cells not only results in increased contamination problems, but also leads to neutralization by breakdown of the additive itself. When additives such as biocide is added to kill the microbes growing in the diesel it will be effective if complete isolation of the tank is possible, else new organisms will grow on the decaying debris of the previous organisms. Moreover regular cleanups are required to remove the decaying bodies of those organisms. In time organisms develop resistance to biocides which will render them ineffective.
2. Materials and Methods
2.1. Preparation of Diesel Samples
Diesel EURO III grade, with no enhanced abilities, purchased from the HP filling station (Jalandhar, Punjab). Sulphur powder was procured from M/s S. D. Fine Chemicals Limited, Mumbai, India.Diesel samples were prepared by varying the sulphur content in each from the range of EURO III to that of EURO II. EURO III Diesel is taken in each of four conical flasks and calculated amount of sulphur is added.Label the samples A, B, C and D. Flasks containing the samples are kept in shaker at 200 rpm and room temperature for 2 hours. The sulphur completely dissolves in the samples to give the required concentration.
2.2. Corrosion Rating of the Samples
Carbon steel Pieces are cut and filed in the manufacturing process laboratory of the institute. Carbon steel used as most of the refinery storage tanks are made of carbon steel (0.6-0.99% carbon).Wipe the Steel Pieces clean and note the weights and dimensions of the carbon steel pieces. Pour 50mL of A, B, C and D diesel samples into a Petri dish and label them.Dip those carbon steel pieces one in each of the petri dishes and cover them with aluminium foil and keep in the incubator at 25°C for 5 days.After 5 days the Steel pieces are wiped with cotton and weighed. The Samples for corrosion rating are shown in Figure 1.

2.3. Preparing of Bushnell Haas Media
Chemicals required for the preparation of Bushnell Haas media are purchased from M/s Merck Chemicals, India. Prepare a mixture of the following chemicals in prescribed amounts:
- MgSO4 0.20g
- CaCl2 0.020g
- KH2PO4 1g
- KH2PO4 1g
- NH4NO3 1g
- FeCl3 0.050g
- Agar 20g
Suspend the 23.27 g mixture in 1000mL of distilled water. Heat and boil to dissolve the medium completely.Sterilize by autoclaving at 15 lbs Pressure (121°C) for 15 min.A white precipitate prior to sterilization turns to ceramic colour in molten form at normal conditions.Petri dishes are cleaned with alcohol and are UV treated in a Laminar Flow Bench for 3 hrs.Molten media in the conical flask is poured into Petri dishes which are UV treated and allowed to set by cooling.
2.4. Inoculation of Fungus and Growth rating
Hormoconisresinae is a fungus commonly found in ATF and Diesel storage tanks. The fungal strain was obtained from IMTECH Chandigarh, India. Molten Bushnell-Haas was poured into petri dish and allowed to cool in a laminar flow bench and the broth is inoculated on to the set media.Petri dishes covered with Aluminium foil are placed in an incubator at 20°C for 5 days, growth is observed for each Petri dish.80mL of Diesel samples earlier prepared are filteredand poured to Tin coated metal cansand the cans are labelled as A, B, C and D representing different concentrations.The Can mouths are covered with Aluminium foils and placed in incubator for 5-10 days. Growth is to be monitored from the 5th day onwards.Diesel samples from cans are each filteredand samples are weighed. The suspended solids are also weighed after drying the filter paper in an oven at 100°C for 16 hrs. The weight of filter paper before and after the filtration is obtained; now after the suspended solids are weighed the weight is reduced from dried filter paper. The Growth of Hormoconisresinaeis shown in Figure 2and theSludge content for various samples are shown in Figure 3.


3. Theory and Calculation
3.1. Corrosion Rating
When a Steel piece is dipped in diesel it gets corroded and it loses weight which will be measured by the difference of initial and final weights.As both the sides of the metal are exposed to diesel the loss in thickness is halved. As the sulphur in the sample increases the corrosion rating also increases.
- Change in volume=change in mass/density
- Density =7.86g/cm3
- Change in thickness (t) = change in volume/surface area
As both the sides of Steel pieces were in contact with diesel for 5 days
R = 365 t
- All the Ratings are in mm/year
The results of Corrosion rating for diesel samples are shown in Table 1.
Table 1. Corrosion rating for diesel samples.
|
Sulphur Content ppm |
Initial mass g |
Final mass in g |
Change in mass |
Change in volume |
Area |
Thickness in cm |
Rating in mm/year |
|
350(blank) |
42.2138 |
42.1808 |
0.033 |
0.004319 |
11.22 |
0.00038501 |
0.14053 |
|
400 |
52.4162 |
52.3502 |
0.066 |
0.008522 |
14.4 |
0.00059186 |
0.21603 |
|
450 |
57.07437 |
56.9684 |
0.10597 |
0.013483 |
13.6 |
0.00099142 |
0.36187 |
|
500 |
49.9652 |
49.8183 |
0.1469 |
0.018697 |
12.25 |
0.00015263 |
0.55710 |
3.2. Loss Due to Corrosion
A tank of capacity 30000kL costs 120million Rupees excluding all the pumps and the piping costs. The average life of a commissioned tank is 20yrs according to manufacturing company for current specification of diesel. In general cases every 10yrs the tanks are subjected to vigorous checkups and the welding and maintenance charges are fairly high, here the life is considered 20years for calculation(Turner,1983). The corrosion ratings are used to obtain the corrosion losses per year.
EURO III diesel has a specification of 350ppm;it gives a corrosion rating of x mm/year
Thickness corroded in 20years T =20 X mm
Time taken to corrode this thickness T for various samples
Yi = 20 X / Xi
- Xi is rating for the diesel samples
- Yi is the time taken in years for the diesel samples to corrode the thickness T.
- It is assumed that the Tank has to be discarded once the Regulation thickness is corroded.
Cost of a tank = 120million Rupees
Loss for 350ppm sample is = 120million
L = 6 (20 / Yi) - 6
L is in millions/year.
As there are about 36 Diesel tanks in a refinery, the total loss/year due to corrosion will be:
Lt = 36L
The results for the total Corrosion Losses for different diesel samples are shown in Table 2 and the results of Microbial Growth Rating in terms of suspended solids are shown in Table 3.
Table 2. Total Corrosion Losses for different diesel samples.
|
Concentration in ppm |
Time in years taken to corrode the thickness(Yi) in years |
Loss in millions/year For a tank/year |
Total Corrosion loss in millions of Rupees/year |
|
350 |
20 |
6.0 |
216.0 |
|
400 |
13 |
9.2 |
332.0 |
|
450 |
7.7 |
15.5 |
556.7 |
|
500 |
5 |
23.8 |
857.1 |
Table 3. Microbial Growth Rating in terms of suspended solids.
|
Sulphur Content (ppm) |
Volume of the sample (mL) |
Mass of the sample |
Weight of suspended solids(g) |
Final loss of diesel (g) |
|
350 Blank |
80 |
67.6 |
0.0656 |
0.2455 |
|
400 |
80 |
67.6 |
0.0413 |
0.1733 |
|
450 |
80 |
67.6 |
0.0.311 |
0.1056 |
|
500 |
80 |
67.6 |
0.0104 |
0.0272 |
3.3. Loss Due to Microbial growth integrated for a Refinery's Capacity
Diesel is a food source of Hormoconisresinae, it breaks down the hydrocarbon and generates sludge there by suspended waste is formed which will result in loss of diesel. The loss in fuel for the experiment is integrated to refinery capacity to obtain the growth losses (Kanal,2006)
- A Refinery has a capacity of 12MMTPA=12*109kg of crude.
- 40% of Capacity is of diesel=4.8MMTPA of Diesel/year.
- Density of the diesel sample is=0.845g/mL
- Weight of the sample= 80*0.845=67.6g
- Cost of diesel: 4.8*1012 gm/0.845=5.680*109 L
- Cost of diesel/L=Rupees 25.00
- So total cost=1.42011*1011Rupees/year
- Consider the contaminated samples:
- X gm of loss in 67.6g of diesel so integrating for 4.8MMPTA
- N=(67.6-X)/67.6
- S=N*4.8*1012 g
- Volume of contaminated sample (V)=S/0.845
- Cost of the sample(C)=(V/1000*0.845)*25
- Loss/year=1.42011*1011-C Rupees/year
The results of Microbial Growth Losses for diesel samples in Refinery capacity are shown in Table 4 and the results of Total Loss for diesel samples in Refinery capacity are shown in Table 5.
Table 4. Microbial Growth Losses for diesel samples in Refinery capacity.
|
Sulphur content in sample in ppm |
Mass of the diesel sample (g) |
Actual mass of diesel present (g) |
Loss in millions of Rupees/year |
|
350 |
67.6 |
67.3545 |
514.9 |
|
400 |
67.6 |
67.4267 |
363.2 |
|
450 |
67.6 |
67.4944 |
221.0 |
|
500 |
67.6 |
67.5728 |
56.3 |
Table 5. Total Loss for diesel samples in Refinery capacity.
|
Sulphur content (ppm) |
Corrosion losses in millions of Rupees/year |
Microbial growth losses in millions of Rupees/year |
Total loss in millions of Rupees/year |
|
350 |
216 |
514.9 |
730.9 |
|
400 |
332.05 |
363.22 |
695.27 |
|
450 |
556.7 |
221.00 |
777.7 |
|
500 |
857.1 |
56.30 |
913.4 |
4. Discussion
4.1. Corrosion Loss with Varying Sulphur Content
The diesel samples with different sulphur content were prepared and the introduction of Carbon steel pieces into the sample gave the corrosion rating (bucker,2011).As the sulphur content in diesel increases the acidic nature of diesel magnifies increasing the corrosion rate of the storage vessel in this case tank thereby increasing maintenance cost and the capital cost of the Unit.
4.2. Growth Loss with Varying Sulphur Content
Microbial growth decreases with increasing sulphur content, as discussed above higher the sulphur more acidic the medium which affects the growth adversely(Hettige,1989). Here as the sulphur increases in diesel fungal growth decreases there by the sludge content formed is decreased. Sludge content decrease denotes less loss of diesel and less clogging leading to less maintenance cost and less product losses
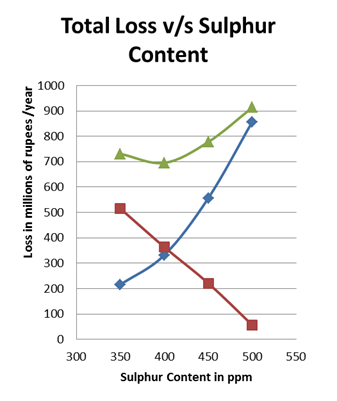
4.3. Total Loss with Varying Sulphur Content
The above two plots denoted the variation of Corrosion and growth loss with sulphur content, these losses add up to total loss. The nature of the two curves are opposite, as the corrosion loss increases with sulphur content product& maintenance loss decreases(Rodriguez,2010). There by plotting the total cost against sulphur content we are able to obtain the sulphur content with lowest total loss which is termed C optimum which ranges from 380-400ppm. The Graph showing variation in Total Losses with Sulphur content is shown in Figure 4.
5. Conclusion
It is observed that by the increase in sulphur content, the Microbial Growth in diesel can be checked and the corrosion due to Microbial Growth can be reduced drastically. But the increase in sulphur content leads to increase in metal corrosion due to sulphur. As the two ratings showed opposite trends, they check on each other. The ratings obtained from the experiments are integrated to refinery capacity. Total loss is determined in refinery terms from the sum of corrosion loss and Microbial Growth loss. Optimum sulphur is that content in which the Total loss is minimal. It ranges from 380 to 400ppm as observed. If microbial growth is the immediate problem of concern the Sulphur content can be kept as 400ppm, if Corrosion is given preference then the Sulphur content can be kept as 380ppm.
References
Aydin, H., Ilkilic, C. (2012). Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods, Fuel, 102, 605-612. View Article
Bücker, F., Santestevan, N. A., Roesch, L. F., Jacques, R. J. S., Peralba, M. C. R., Camargo, F. A. O., Bento, F. M. (2011). Impact of biodiesel on biodeterioration of stored Brazilian diesel oil. International Biodeterioration & Biodegradation, 65, 172-178. View Article
Gaylarde, C. C., Bento, F. M., Kelley, J. (1999). Microbial contamination of stored hydrocarbon fuels and its control. Revista de Microbiologia 30, 01-10. View Article
Hartman, J., Geva, J., Fass, R. A. (1991).Computerized expert system for diagnosis and control of microbial contamination in jet fuel and diesel fuel storage systems."Proc. of 4thIntl. Conf. on Stability and Handling of Liquid Fuels", Orlando, Fa, Nov. 19-22, pp.153-166.
Hettige, G. E. G., Sheridan, J. E. (1991).Effect of biocide on microbiological growth in middle distillate fuels, International Biodeterioration, 25, 175-189. View Article
Hill, E. C. Hill, G. C. (1993).Microbiological problems in distillate fuels. Trans. Inst. Marine Eng., 104, 119-130. View Article
Kanal, R. A., Hur, H. G. (2006). Growth of Phanerochaetechrysosporium on diesel fuel hydrocarbons at neutral pH. Chemosphere, 63, 202-211. View Article
Lin, J., Gaustad, G., Trabold, T. A. (2013). Profit and policy implications of producing biodiesel-ethanol-diesel fuel blends to specification, Applied Energy, 104, 936-944 View Article
Muthukumar, N., Maruthamuthu, S., Palaniswamy, N. (2007). Role of cationic and nonionic surfactants on biocidal efficiency in diesel-water interface, Colloids and Surfaces B: Biointerfaces, 57,152-160. View Article
McClellan, R. O., Hesterberg, T. W., Wall, J. C. (2012). Evaluation of carcinogenic hazards of diesel engine exhaust needs to consider revolutionary changes in diesel technology, Regulatory Toxicology and Pharmacology, 63, 225-258. View Article
Rajasekar, A., Maruthmuthu, S., Palaniswamy, N., Rajendram, A. (2007). Biodegradation of corrosion inhibitors and their influence on petroleum product pipeline, Microbiological Research, 162, 355-368. View Article
Rodriguez, R. C. E., Rodriguez, E., Blanco, R., Cordero, I., Segura, D. (2010).Fungal contamination of stored automobile-fuels in a tropical environment. Journal of Environmental Sciences, 22, 1595-1601. View Article
Sharafutdinov, I., Stratiev, D., Shishkova, I., Dinkov, R., Petkov, P. (2012), Industrial investigation on feasibility to raise near zero sulphur diesel production by increasing fluid catalytic cracking light cycle oil production, Fuel Processing Technology, 104, 211-218 View Article
Turner, A. P. F., Eaton, R. A., Jones, E. B. G. (1983). Nutritional aspects of ship fuel system contamination by CladosporiumResinae In: Oxley TA and Barry S, editors. Biodeterioration 5, eds. London.Wiley and Sons; pp. 507-516. View Article

