Volume 1 - Year 2012 - Pages 7-12
DOI: 10.11159/ijepr.2012.002
Formation of Trihalomethanes (THMs) during Chlorination of Landfill Leachate
Nanzhu Li, Yang Deng*
Department of Earth and Environmental Studies
Montclair State University
1 Normal Ave, Montclair, New Jersey, United States
lin3@mail.montclair.edu, dengy@mail.montclair.edu
Abstract - Co-treatment of landfill leachate and sewage in a traditional wastewater treatment plant (WWTP) is a very common leachate management method in the United States. However, biologically recalcitrant and humic-like leachate organic matters (the major fraction of mature leachate organics) cannot be truly removed by the aerobic biodegradation in WWTPs. Prior to effluent discharge from WWTPs, chlorine for disinfection may largely transform the humic-like substances to various disinfection by-products (DBPs). In this study, formation potential (FP) of trihalomethanes (THMs) (the most common DBP type) during leachate chlorination was evaluated. Seven-day DBP FP tests were conducted to measure THM formation potential (THMFP) of a typical mature leachate and a representative secondary effluent. Results show that the THMFP of the tested leachate was 20,227 ppb, much greater than that (180 ppb) of a WWTP secondary effluent. Different molecular weight (MW) leachate organics fractions exhibited different FP patterns. Most of THMs (65%) was formed from the lowest MW organics groups (<1,000 Da); however, the THMFP/DOC for the < 1,000 Da group was the lowest (62 ppb/ppm), in comparisons with 222, 258, and 261 ppb/ppm of 1,000-10,000, 10,000-100,000, and >100,000 Da leachate organics. The results demonstrated that WWTP co-treatment of leachate should be carefully selected in the areas that depend heavily upon water reuse or where treated wastewater accounts for a high fraction of downstream drinking water plant's raw water.
Keywords: Disinfection, Disinfection By-Products (DBPs), Landfill Leachate, Trihalomethanes (THMs), Formation Potential (FP)
© Copyright 2012 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
1. Introduction
The protection and restoration of our natural and living environment are pivotal for the sustainable development of the United States (US). Over the past four decades, landfill has been the primary solid waste disposal method in the United States and many other countries (US Environmental Protection Agency, 2010). In 2009, 54.3 % of 243-million tons of solid wastes generated in the United States was landfilled. In the State of New Jersey (NJ) in the US, there are 13 active landfills that are disposing of approximately 50% of solid wastes generated every year, as well as ~500 closed or inactive landfills (Ezyske and Deng, 2012). After precipitation percolates through waste deposited in landfills, landfill leachate, a highly contaminated wastewater produced from the landfills, is produced. As a result of gravity, landfill leachate tends to accumulate at the bottom of landfills.
Chemical characteristics of landfill leachate are extremely variable due to variability in generation rates over a broad time frame, non-uniformity of waste decomposition with respect to time and space, and impacts related to environmental conditions and anthropogenic factors (Vagliasindi, 1995). Landfill leachate characteristics rely heavily upon landfilling age, waste nature, moisture availability, temperature, pH, depth fills, compaction and other factors (US Environmental Protection Agency 1995; Viraraghavan and Singh 1997). Of principal concern within leachates are organic constituents, along with ammonia and heavy metals such as arsenic (Beccaloni, et al., 2000; Ghosh et al., 2004). Although composition and strength of leachate organic matters are significantly site-specific, the trend of their variation with respect to landfilling time is roughlyconstant. With the increasing landfill age and humification, most of the organic compounds become non-biodegradable humic-like substances (Kjeldsen et al., 2002). The leachate organic strength is generally a few tens to thousands fold of sewage strength.
For old landfills that were not designed with any leachate collection and liner system, the leachate produced there may freely escape into underlying soil and groundwater to cause local contamination. In fact, landfill leachate has been a major soil and groundwater contamination source in history. For example, leachate is responsible for pollution occurring in 45 of 146 New Jersey Superfund sites (Ezyske and Deng, 2012). For modern landfills that have unique liner and leachate collection systems, landfill leachate can be pumped out for further treatment prior to discharge. Currently, the major leachate treatment method in the United States is delivery of landfill leachate to traditional local wastewater treatment plants (WWTPs) for co-treatment of landfill leachate and sewage.
Because the governing mechanism of WWTPs for removal of organics is aerobic biological degradation, the major fraction of leachate humic organics is not truly decomposed due to their non-biodegradability (Deng, 2009). Prior to effluent discharge from WWTPs, chlorine applied in the last disinfection step has an extremely high potential to transform the humic-like substances to a variety of disinfection by-products (DBPs), including EPA regulated trihalomethanes (THMs) and haloacetic acids (HAAs), and unregulated N-containing DBPs such as haloacetamides (HAcAms) and N-Nitrosodimethylamine (NDMA) (Chu et al., 2010, 2012). Many DBPs are cancerogenic, mutagenic, and/or genotoxic. Because treated wastewater represents a significant fraction of water flow in many US rivers, discharge of leachate into WWTPs can result in the exposure of humans and aquatic organisms to the DBPs. Much worse, the drinking water supply of some communities is withdrawn from rivers subjected to significant upstream wastewater effluent discharges, but they poorly remove the emerging organic pollutants. Therefore, quantification of the formation potentials of DBPs contributed from leachate organic matters is vital. However, to the best of our knowledge, there has not been a systematic study on the topic.
Total trihalomethanes (TTHMs) are chemical compounds in which three of the four hydrogen atoms of methane (CH4) are replaced by halogen atoms, including chloroform, bromodichloromethane, dibromochloromethane, and bromoform. Among them, chloroform and dibromochloromethane are carcinogens; and another THM, bromodichloromethane, has been identified as a mutagen (Grossman, 1999). Mutagens are considered to affect the genetics of future generations in addition to being carcinogenic. A California study indicates that THMs may be responsible for reproductive problems and miscarriage. The study found a miscarriage rate of 15.7 percent for women who drank five or more glasses of cold water containing more than 0.075 mg/l TTHM, compared to a miscarriage rate of 9.5 percent for women with low TTHM exposure (Madabhushi, 1999). Besides, TTHMs are linked to bladder cancer, heart, lungs, kidney, liver, and central nervous system damage. United States Environment Protection Agency (US EPA) has published the Stage 1 Disinfectants/Disinfection Byproducts Rule to regulate TTHM at a maximum allowable annual average level of 80 parts per billion.
The objective of this study is to quantify the formation potential of THMs, a well-known DBP type, during chlorination of landfill leachate, and determine the contributions of different molecular weight (MW) organics in leachate to the formation of THMs. To the best of our knowledge, this study would be the first scientific effort in this topic.
2. Materials and Methods
2. 1 Reagents
All the chemicals were at least analytical grade, except as noted. All the chemical solutions were prepared with ultrapure water produced from a Milli-Q Academic water purification system. Landfill leachate samples were collected from the leachate holding tank operated by New Jersey Meadowland Commissions in Kearny, New Jersey, United States. The leachate is a mixture of leachates that were pumped from Landfill 1-A and Landfill 1-E, of which both received municipal non-hazardous solid waste. Secondary effluent was collected from the outlet of a secondary sedimentation tank in the Joint Meeting of Essex & Union Counties Wastewater Treatment Plant in Elizabeth, New Jersey, United States. The secondary effluent sample was not chlorinated. Once collected in clean glass bottles, the landfill leachate or secondary effluent samples were immediately transported to the Geochemistry Laboratory at Montclair State University, and stored in a refrigerator at 4°C prior to use.
2. 2 Experimental Procedure
Seven-day THM formation potential (THMFP) tests were conducted to quantify the maximum capacity of THM formation from the landfill leachate or secondary effluent samples under different experimental conditions. In the tests for the leachate THMFP, the leachate was diluted by ultrapure water from a Milli-Q water purification system with different dilution factors (1:2, 1:10, 1:20, 1:40, 1:100, and 1:400). In the tests for THMFP of secondary effluent, the secondary effluent was diluted by ultrapure water with a dilution factor of 2 – 10. In order to minimize the influence of particles in the samples, the leachate or secondary effluent was filtered by 0.45 µm membrane filters before THMFP tests. The THMFP test procedure followed the Standard Method 5710 Formation of Trihalomethanes and Other Disinfection By-Produces (APHA, AWWA and WEF, 1995). To ensure that the chlorine added was adequate to react with all the organic matters in the landfill leachate or secondary effluent, the chlorine dose was estimated using the following equation (Chu et al., 2009).
| 2=3DOC+7.4NH3-N+10 | (1) |
Where,
Cl2, chlorine dose (mg/L);
DOC, dissolved organic carbon of the leachate (mg/L)
NH3-N, ammonia nitrogen of the leachate (mg/L)
In the above equation, the amounts of chlorine consumed for reactions with organic matters and ammonia nitrogen, as well as certain amount of excess chlorine, are considered. THMs produced in the formation potential (FP) tests were measured using the HACH kits (Method 10224). DOC was tested using the HACH Total Organic Carbon (TOC) Reagent Set (0.3-20 mg/L).
In the tests to study the effects of molecular weight (MW) on THMFP, leachate organics were fractionated into different MW ranges using a Millipore stirred ultrafiltration cell (Model 8050). Certain amount of landfill leachate was stored in the stirred cell installed with a 100,000 Da ultrafiltration membrane filter. The leachate was filtered through the membrane filter under a high N2 pressure. The organic molecules in the filtrate only contained organic compounds with a MW range of 0 -100,000 Da. Similarly, the MW ranges of the leachate organic matters could be further fractioned. The tested MW ranges included 0-1,000 Da, 1,000-10,000 Da, 10,000-100,000 Da, and > 100,000 Da. All the tests were run in duplicates, at least. Symbols and error bars in the figures represent the algebraic mean values of and standard deviations of the data obtained.
3. Results
3.1 Characterization of Landfill Leachate
Basic physical and chemical characteristics of the landfill leachate samples are shown in Table 1. As seen, the leachate sample was characterized by a weakly alkaline solution pH (8.53) and a low BOD5/COD (<0.2), thus indicating that the leachate was typically mature and the leachate organic matters were highly biologically recalcitrant. The COD range (784-824 mg/L) suggested a much higher organic content than that of a typical sewage. In addition, 400 mg/L ammonia nitrogen was significantly greater than the ammonia nitrogen found in sewage. The ammonia nitrogen could consume certain chlorine added during the THMFP tests as shown in Reactions (2) to (5).
| Cl2 + H2O = HClO + HCl | (2) |
| HClO + NH3 = NH2Cl + H2O | (3) |
| HClO + NH2Cl = NHCl2 + H2O | (4) |
| HClO + NHCl2 = NCl3 + H2O | (5) |
Table 1. Basic physical and chemical characteristics of the landfill leachate sample.
| Parameters | Value |
| pH | 8.53 |
| DOC (mg/L) | 240 |
| COD (mg/L) | 784 - 824 |
| BOD5/COD | < 0.2 |
| Ammonia nitrogen (mg/L) | 400 |
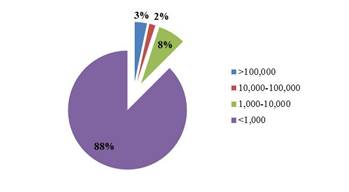
Fractions of different MW leachate organics are shown in Fig. 1. The highest DOC fraction was the 0 - 1,000 Da leachate organics group corresponding to 88% of the total DOC, followed by 1,000-10,000 Da MW organics (8%), thus indicating that most of organics in the tested leachate were low MW compounds. In contrast, 10,000-100,000 and > 100,000 Da organics groups only accounted for 2% and 3% of the total DOC, respectively. A high fraction of low MW organic molecules are most likely because high MW organic compounds were more readily removed within landfills as a result of adsorption and screening by solid waste. Moreover, the findings also demonstrate that the low MW organic products after anaerobic degradation within landfills were highly refractory with a low BOD5/COD and unsuitable for treatment in traditional wastewater treatment plants in which the aerobic degradation is the governing mechanism.
3.2 THM Formation Potential
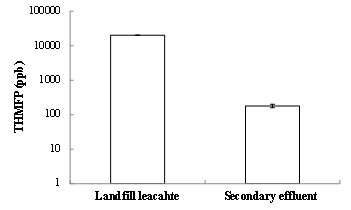
THMFPs of the landfill leachate and the secondary effluent are shown in Fig. 2. The THMFP of the landfill leachate was 20,227 ppb, much greater than that of the secondary effluent (180 ppb). Such a high THMFP found in the leachate sample may be due to two reasons. First, the leachate characterized with a high DOC (784-824 mg/L) had much more organic molecules than raw water in drinking water treatment plants or secondary effluent from wastewater treatment plants. In particular, previous studies (Singer, 1994) demonstrated that organic matters in mature leachate tended to be more humic, and the humic-like organic substances were typical THM precursors. Second, the leachate organics had a much higher THM/DOC than secondary effluent (discussed later).
Compared with the US EPA Maximum Contaminant Level (MCL) of THM (80 ppb), the THM produced from the landfill leachate sample was extremely high. This finding suggests that the THM formation from a typical mature landfill leachate was significant, and may contribute to a high concentration of THMs in the treated wastewater effluent after it is co-treated with sewage in a traditional wastewater treatment plant.
3.3 Ratio of THMFP to DOC (THMFP/DOC)
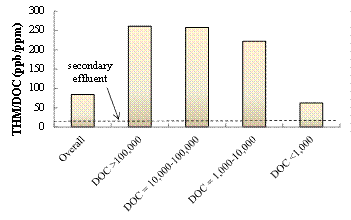
THMFPs of the secondary effluent and landfill leachate samples at different MW leachate organics groups are shown in Fig. 3. The secondary effluent exhibited an appreciably low THMFP/DOC (12 ppb THM/ppm DOC) relative to those of the landfill leachate. In contrast, the overall THMFP/DOC of the leachate sample was 84 ppb THM/mg DOC. Furthermore, the two highest THMPF/DOC ratios (261 and 258 ppb THM/ppm DOC) were observed in the >100,000 Da and 10,000-100,000 Da groups, respectively. The 1,000-10,000 Da group had a slightly lower THMFP/DOC (222 ppb THM/ppm DOC). However, the lowest MW organic group (0-1,000 Da) had the lowest THMPF/DOC (63 ppb THM/ppm DOC). Therefore, generally speaking, the THMFP/DOC decreased with the increasing MW, thus implying that high MW leachate organic compounds had a much high potential to transform to THMs under the reactions with chlorine. Of note, the low overall THMFP/DOC is primarily due to an extremely high fraction (88%) of 0-1,000 Da leachate organic molecules that were characterized with the lowest THMFP/DOC.
4. Discussion
Co-treatment of landfill leachate in a local traditional wastewater treatment plant is a very common practice in solid waste management in the United States. For example, almost all the landfill leachates in the State of New Jersey, United States, are delivered for co-treatment in local WWTPs. This option adequately takes advantages of existing treatment facilities, so that leachate disposal costs are substantially reduced. However, the wastewater facilities were not specially designed to address solid waste-derived contaminants from landfill leachate. In particular, refractory leachate organic compounds are rarely degraded in wastewater treatment plants due to a low biodegradability. Previous studies (Reinhart and Grosh, 1997; Deng, 2009) have revealed that the fraction of humic substances gradually becomes dominant with time in mature leachates. The relative abundance of organic compounds present in landfill leachate is observed to decrease with landfilling time in the following order: free volatile fatty acids, low molecular weight aldehydes, amino acids, carbohydrates, peptides, humic acids, phenolic compounds, and fulvic acids (Reinhart and Grosh, 1997). In numerous studies (Chu et al., 2009, 2010, and 2011) to evaluate the formation of DBPs in drinking water treatment, humic acids, as well as fulvic acids, have been frequently demonstrated to be the major precursors of DBPs. Therefore, the formation of DBPs from chlorination of non-biodegradable leachate organic matters during co-treatment of landfill leachate and sewage may be a great concern in engineering practice.
In this study, we have found that the overall THM produced during direct chlorination of a typical mature landfill leachate was 20,227 ppb, 112 times as the THM produced from chlorination of a representative secondary effluent. In practice, landfill leachate may be highly diluted by sewage in a wastewater treatment plant, so that the actual concentration of THM produced is substantially lowered. However, under some situation (e.g. subsequent to heavy rainfalls when much landfill leachate is generated), the amount of landfill leachate relative to that of sewage may be significantly increased so that the leachate should not be ignored during treatment. In many countries, federal, regional, or state agencies typically set a maximum volumetric ratio of landfill leachate to sewage for the co-treatment practice to minimize the disturbance of landfill leachate on the wastewater treatment performance (e.g. too high level ammonia may reduce the population of beneficial microbes due to its toxicity in aerobic oxidation reactors). The maximum ratio is often set at 5% (e.g. in China). If 5% is used for this study, the final THMFP during chlorination of the mixed leachate and secondary effluent samples was over 1,000 ppb, substantially greater than the THM limit level (80 ppb) in drinking water. Although the treated wastewater is not used for the purpose of direct drinking water production, the THM produced may negatively impact the environment of receiving water bodies (e.g. microbial population and species), or may be an issue in the areas that heavily rely upon water reclamation using the secondary effluent as a major reclaimed water source.
Besides THMs, many other DBPs may be also produced accompanied with co-treatment of landfill leachate and sewage. HAAs are the other well-known and EPA-regulated DBP feedback. Compared with THM, HAAs are much more concerned, because they are non-volatile, and may keep in the treated wastewater for a long time. Another concern includes some emerging N-containing DBPs, such as HAcAms and NDMA. The traceable but highly toxic N-DBPs may be produced in the presence of chlorine, ammonia, and complex leachate organic matters in the leachate matrix. Formation of other DBPs during chlorination of landfill leachate will be further evaluated in our future research plan.
To minimize the DBPs formation from the organic matters of landfill leachate in treated wastewater effluent, certain strategies should be considered. One strategy is to remove the DBP precursors (the humic-like landfill leachate organic compounds) prior to chlorination in wastewater treatment plants. Due to their biologically recalcitrant properties, the organic matters should be removed by physicochemical processes. Reviews on physical and chemical methods for removal of refractory landfill leachate organics are available elsewhere (Wiszniowski et al., 2006; Deng, 2007; Renou et al., 2008). Several treatment methods, including hydroxyl radical (OH•)-based advanced oxidation processes (AOPs) (Wang et al., 2003; Deng, 2009), electrochemical oxidation (Deng and Englehardt, 2007), and membrane processes (Van Der Bruggen et al., 2003; Deng, 2007), have gained much attention because of their high treatment capacities. OH• -based AOPs typically remove landfill leachate COD by 43-77% (Deng, 2009). The treatment efficiencies depend upon the methods to produce hydroxyl radicals (e.g. Fenton reaction, H2O2/O3, and UV/H2O2), and experimental conditions (e.g. pH). Electrochemical oxidation can oxidize almost 100% of COD, as well as ammonia nitrogen. However, a high cost in energy consumption, electrode poisoning, and reactor scale-up may significantly restrict its application. Pressure-driven membrane processes, nano-filtration (NF) and reverse osmosis (RO) in particular, are able to remove almost all the organic and inorganic impurities in landfill leachate including organic matters. But application of NF and RO may be limited by high capital and operation costs because of high energy consumption and membrane replacement due to membrane fouling. Recently, some emerging landfill leachate treatments have been studied. For example, sulfate radical (SO42- ● )-based advanced oxidation processes have been demonstrated to simultaneously remove > 90% of COD and 100% of ammonia (Deng and Ezyske, 2011). Another strategy is that appropriate tertiary treatment subsequent to disinfection should be selected to remove DBPs produced. Many advanced wastewater treatment processes have been proposed to reduce DBPs, such as adsorption (Sakoda et al., 1991), chemical oxidation (Tang and Tassos, 1997; Shemer and Narkis, 2005), and membrane processes (Waniek et al., 2002).
5. Conclusion
This study was the first scientific effort to quantify the formation of THMs during chlorination of a mature landfill leachate. Results showed that the refractory and humic like leachate organic matters during chlorination were transformed to an extremely high level of THMs, substantially greater than the THM yield during chlorination of secondary effluent. Furthermore, high MW leachate organic matters exhibited high THMFP/DOC, though their fractions in the leachate DOC were relatively low. Our findings demonstrate that THM formation during co-treatment of landfill leachate and sewage should be carefully considered in solid waste management and wastewater treatment practices, particularly in the areas that depend heavily upon water reuse or where treated wastewater accounts for a high fraction of downstream drinking water plant's raw water. Different strategies are recommended to reduce THMs produced in treated wastewater effluent. Appropriate physicochemical treatment processes may be selected to remove the THM precursors (refractory leachate organic matters) prior to disinfection, or certain tertiary treatment is considered to remove THMs produced.
Acknowledgements
This project is sponsored by New Jersey Water Environmental Association (NJWEA). Special thanks to Mr. Tom Marturano and Mr. Nick Marucci at New Jersey Meadowland Commissions for leachate collection.
References
APHA, AWWA, and WED (1995). Standard Methods for the Examination of Water and Wastewater, 19th edition, APHA Publication Office. View Article
Van Der Bruggen, B., Vandecasteele, C., Van Gestel, T., Doyen, W., Leysen, R. (2003) A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environmental Progress, 22 (1), 46–56. . View Article
Beccaloni, E., Borrello, P., Musmeci,L., Stacul, E. (2000) Arsenic and Heavy Metals in Leachate from a Real Landfill and a Laboratory Landfill, Annali Di Chimica, 90(11-12), 629-636. View Article
Chu W., Gao N., Deng Y., Dong B. (2009) Formation of chloroform during chlorination of alanine in drinking water. Chemosphere, 77(10), 346-1351. View Article
Chu W., Gao N., Deng Y. (2010). Screening the precursors of emerging nitrogenous disinfection by-product haloacetamides. Environmental Science and Technology, 44 (10), 3908–3912.
Chu W., Gao, N., Deng Y., Templeton M. R., Yin D. (2011) Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Bioresource Technology, 102, 1116-11166. View Article
Deng, Y. (2007) Physicochemical Removal of Organic Contaminants in Municipal Landfill Leachate, Chapter 1 in Landfill Research Focus by Ernest C. Lehmann (Editor), Nova Science Publishers, Inc. View Article
Deng, Y., Englehardt, J. (2007) Electrochemical Oxidation for Landfill Leachate Treatment. Waste Management, 27(3), 380-388. View Article
Deng Y. (2009). Advanced oxidation processes (AOPs) for reduction of organics in mature landfill leachates: a review. International Journal of Environment and Waste Management, 4(3-4), 366-384. View Article
Deng., Y., Ezyske, C. (2011) Sulfate Radical-Advanced Oxidation Process (SR-AOP) for Simultaneous Removal of Refractory Organic Contaminants and Ammonia in Landfill Leachate. Water Research, 45, 6189-6194. View Article
Ezyske C., Deng Y. (2012). "Landfill Management and Remediation Practices in New Jersey, United States", Chapter 9 in Management of Organic Waste by Sunil Kumar and Ajay Bharti (Editors), InTech Publisher. View Article
Ghosh, A., Mukiibi, M., Ela, W. (2004) TCLP Underestimates Leaching of Arsenic from Solid Residuals under Landfill Conditions, Environmental Science & Technology, 38(17), 4677-4682. View Article
Grossman, R. (1999). "Tap Water: The Last Taboo." View Article
Kjeldsen P., Barlaz M.A., Rooker A.P., Baun A., Ledin A., Christensen T.H. (2002). Present and long term composition of MSW landfill leachate: a review. Critical Review on Environmental Science and Technology, 32 (4), 297–336. View Article
Madabhushi, B. S. (1999) "What are trihalomethanes?" View Article
Reinhart D.R., Grosh C.J. (1997) Analysis of Florida MSW Landfill Leachate Quality Data, Technical Report, Hinkely Center for Solid and Hazardous Waste Management, Florida, USA. View Article
Renou, S., Givaudan, J. G., Poulain, S., Dirassouyan, F., Moulin, P. (2008) Landfill leachate treatment: Review and opportunity. Journal of Hazardous Materials, 150 (3), 468–493. View Article
Sakoda, A., Suzuki, M., Hirai, R., Kawazoe, K. (1991). Trihalomethane adsorption on activated carbon fibers. Water Research, 25(2), 219–225. View Article
Shemer, H., Narkis, N. (2005) Trihalomethanes Aqueous Solutions Sono-oxidation. Water Research, 39 (12), 2704–2710. View Article
Singer, P. C. (1994) Control of Disinfection By‐Products in Drinking Water. Journal of Environmental Engineering, 120, 727–744. View Article
Tang, W. Z., Tassos, S. (1997) Oxidation kinetics and mechanisms of trihalomethanes by Fenton's reagent. Water Research, 31 (5), 1117–1125. View Article
Waniek, A., Bodzek, M., Konieczny, K. (2002).Trihalomethane Removal from Water Using Membrane Processes. Polish Journal of Environmental Studies, 11 (2), 171-178. View Article
Wang, F., Smith, D. W., Gamal El-Din, M. (2003) Application of Advanced Oxidation Methods for Landfill Leachate Treatment – A Review. Journal of Environmental Engineering and Science, 2(6), 413-427. View Article
Wiszniowski, J., Robert, D., Surmacz-Gorska, J., Miksch, K., Weber, J. V. (2006) Landfill Leachate Treatment Methods: A Review. Environmental Chemistry Letters, 4(1), 51-61. View Article

